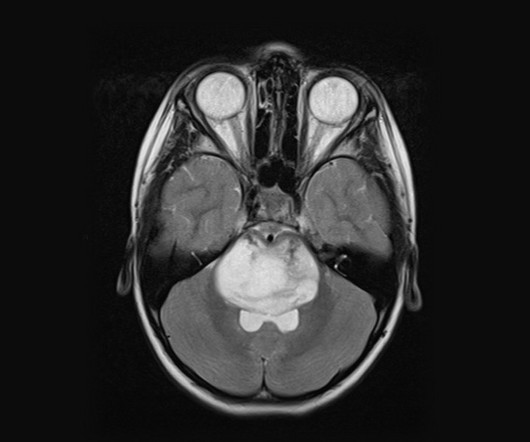
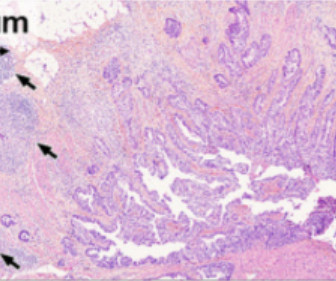
POLB 001: tackling cytokine storms before they start
Drug Target Review
MAY 23, 2025
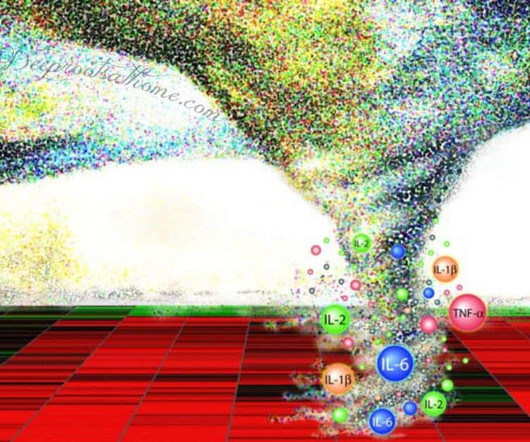
In the ever-evolving landscape of immuno-oncology, managing the side effects of advanced therapies has become just as important as enhancing their efficacy. Our goals are to progress our pipeline and position each asset for clinical and commercial success. Tremble highlights the delicate balance that POLB 001 aims to strike.



































Let's personalize your content