Toxicology transformed: Why accuracy now leads the way
Drug Target Review
DECEMBER 11, 2024
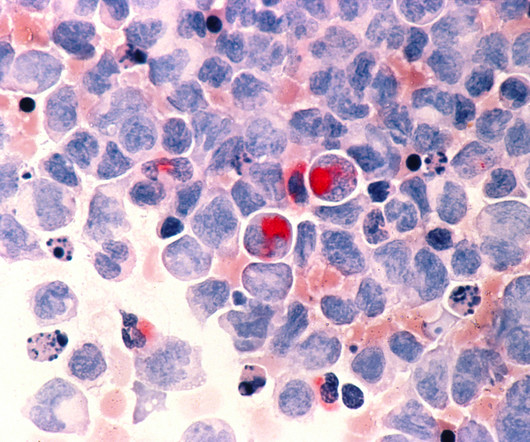
This shift in focus is especially critical in toxicology, where accurate target analysis plays a vital role in identifying toxic effects and ensuring patient safety, particularly as the field transitions from traditional drugs to the promising realm of biotherapeutics, especially for rare diseases.













































Let's personalize your content