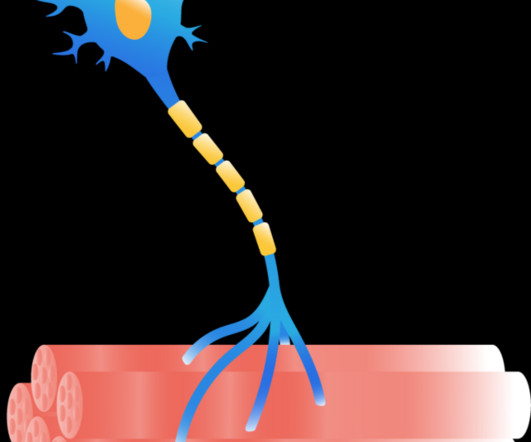
FDA Returns Disappointing News for ALS Stem Cell Therapy
PLOS: DNA Science
OCTOBER 19, 2023
The only treatment for ALS is a drug, Riluzole , taken as a tablet or a film. The amazing testimonials we have seen online do not align with the data that BrainStorm has shared with us or has been published in peer-reviewed publications. However, the clinical course of ALS is the same, whether the cause is familial or not.

























Let's personalize your content