Study strengthens link between shingles vaccine and lower dementia risk
Science Daily: Pharmacology News
APRIL 2, 2025
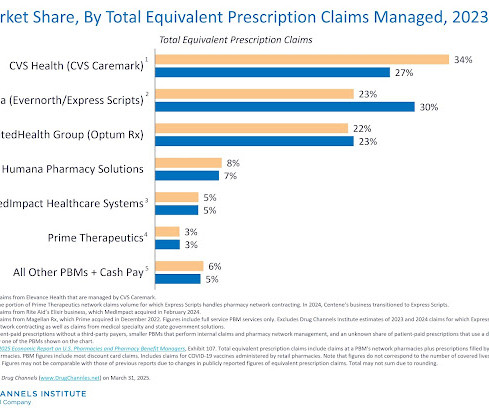
A new analysis of a vaccination program in Wales found that the shingles vaccine appeared to lower new dementia diagnoses by 20% -- more than any other known intervention.

































Let's personalize your content