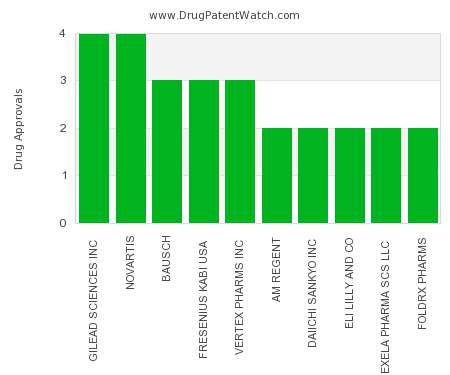
The value of method of use patent claims in protecting your therapeutic assets
Drug Patent Watch
APRIL 5, 2022
This is a guest post by Ray Miller and Megan E. Bowers, Ph.D. at DLA Piper. Ray may be contacted at raymond.miller@dlapiper.com. When it comes to protecting therapeutic assets, I…. The post The value of method of use patent claims in protecting your therapeutic assets appeared first on DrugPatentWatch - Make Better Decisions.






















Let's personalize your content