Biogen Advances Investigational SMA Therapy to Registrational Trials After Positive Phase 1 Data
The Pharma Data
JUNE 25, 2025
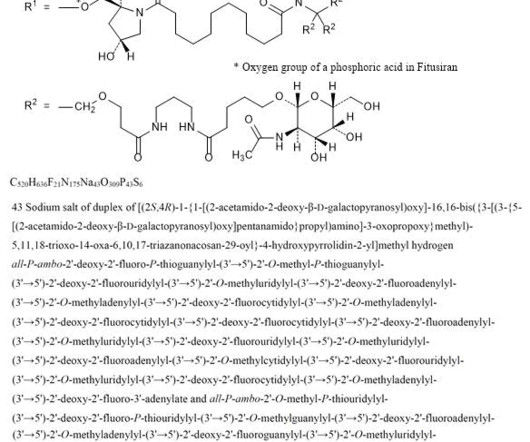
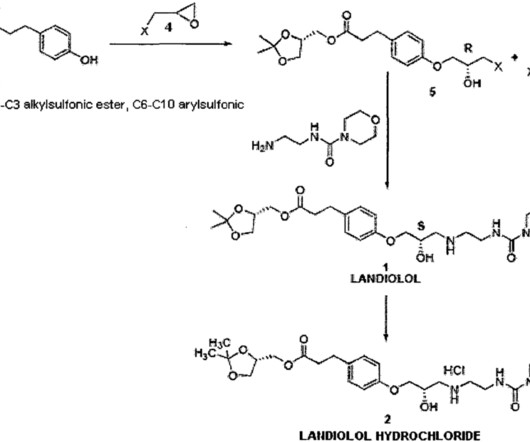
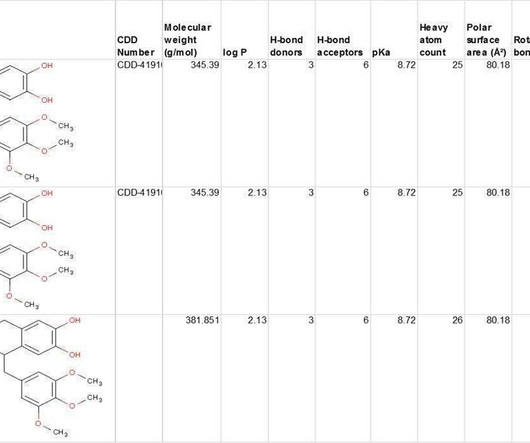
Biogen Reports Promising Interim Phase 1 Results for Salanersen in Spinal Muscular Atrophy, Prepares for Registrational Trials Biogen has announced encouraging topline results from its Phase 1 clinical trial evaluating salanersen (BIIB115/ION306) , an investigational antisense oligonucleotide (ASO) therapy for spinal muscular atrophy (SMA).






































Let's personalize your content