Inavolisib
New Drug Approvals
APRIL 25, 2025
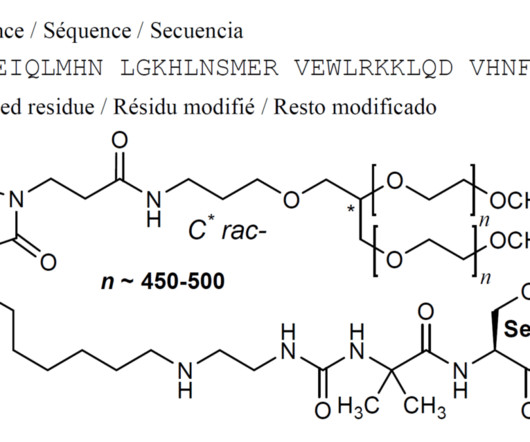
3] Inavolisib was approved for medical use in the United States in October 2024. [3] 19] Society and culture Legal status In October 2024, the US Food and Drug Administration (FDA) approved inavolisib for the treatment of PIK3CA -mutant breast cancer based on the results from the INAVO120 trial. [3] 3 November 2006. cd-21-0072.




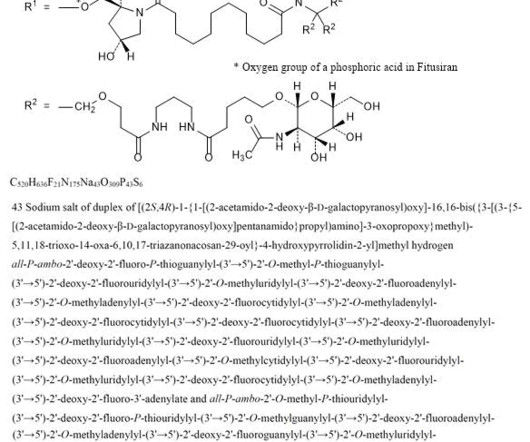

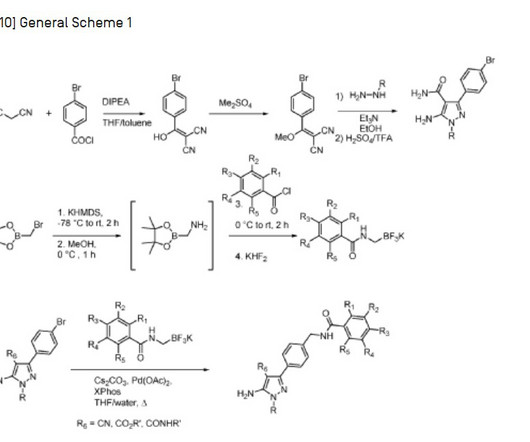
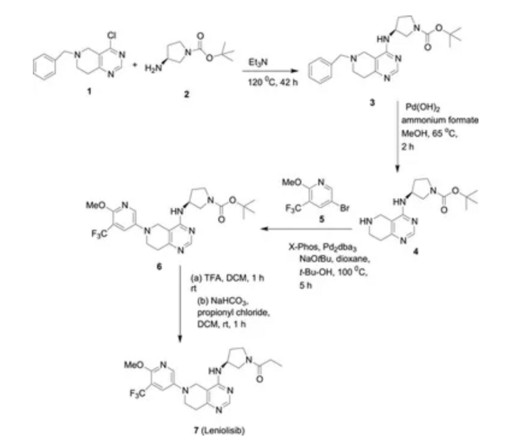
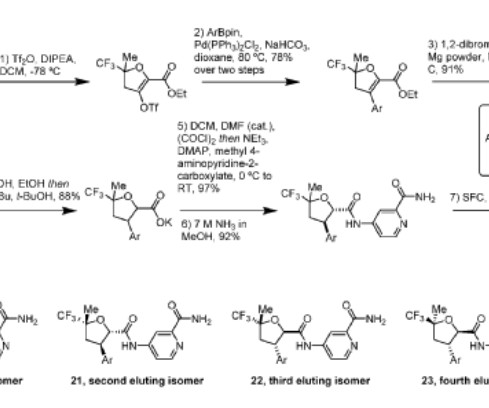





































Let's personalize your content