How do patent thickets vary across different countries
Drug Patent Watch
DECEMBER 10, 2024
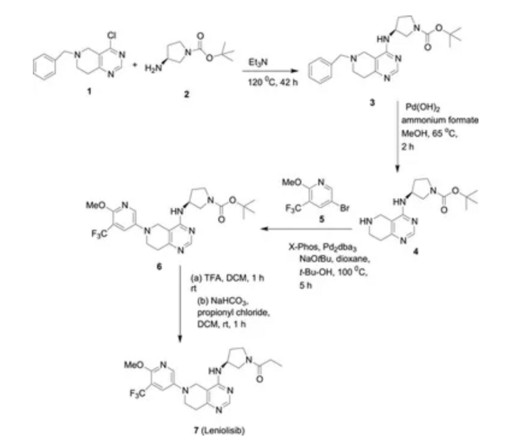
Understanding Patent Thickets: A Global Challenge Before we dive into the international landscape of patent thickets, let’s first grasp what they are and why they matter. Cross-licensing agreements: To navigate patent thickets, many companies enter into cross-licensing agreements.









































Let's personalize your content