From siloed data to breakthroughs: multimodal AI in drug discovery
Drug Target Review
JUNE 11, 2025
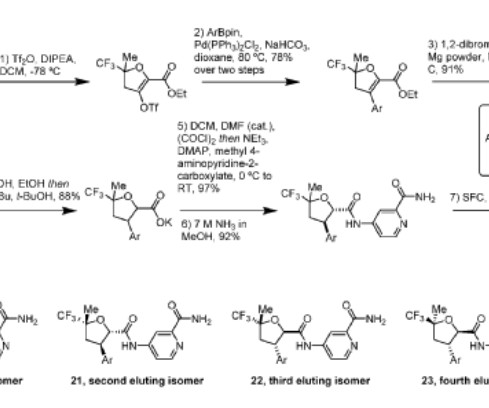
Multimodal language models Generative AI models (GenAI) in the pharmaceutical field have reached the highest level of attention with the Nobel Prizes to Demis Hassabis and John Jumper for AlphaFold, which can predict protein structures. Traditional drug discovery data architecture is manual, messy, proprietary and inflexible.











































Let's personalize your content