How GPCR-targeting therapies are advancing the fight against inflammatory disease
Drug Target Review
JUNE 16, 2025
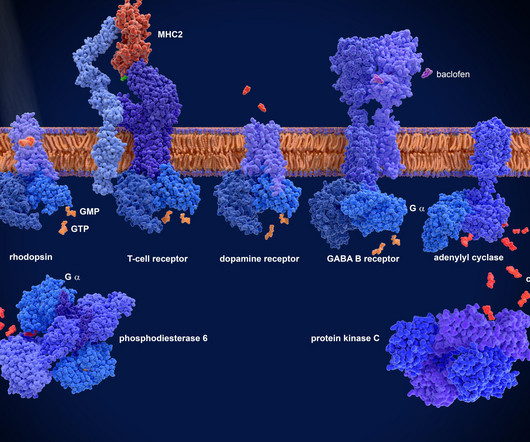
Targeting GPCRs to fight inflammatory diseases GPCR-targeting drugs are well known therapies for a range of disease types, including cardio-metabolic, central nervous system and endocrinological disorders. It also holds potential for neuroinflammatory conditions such as certain types of migraine.















































Let's personalize your content