Drug Discovery Industry Roundup with Barry Bunin — March 1, 2024
Collaborative Drug
MARCH 1, 2024
FDA Approved 55 New Molecular Entities in 2023 Can Ancient Skeletons Give Clues to Modern Medical Mysteries? and More
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Collaborative Drug
MARCH 1, 2024
FDA Approved 55 New Molecular Entities in 2023 Can Ancient Skeletons Give Clues to Modern Medical Mysteries? and More
Collaborative Drug
OCTOBER 7, 2024
This powerful technology is used in drug discovery to guide the acquisition or synthesis of desirable new compounds, as well as to further characterize existing molecules. toxicity or poor bioavailability) Save time and money by using computer-aided drug design Deciding which compounds to synthesize next can be a challenging question.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

FDA Law Blog: Drug Discovery
NOVEMBER 10, 2024
Food and Drug Administration (FDA) plays a pivotal role in fostering the development of treatments for rare diseases through its Orphan Products Grants Program. Each year, FDA selects a limited number of clinical trials to fund to help sponsors pursue development of medical products for rare diseases and advance their field.
Metabolite Tales Blog
JANUARY 26, 2023
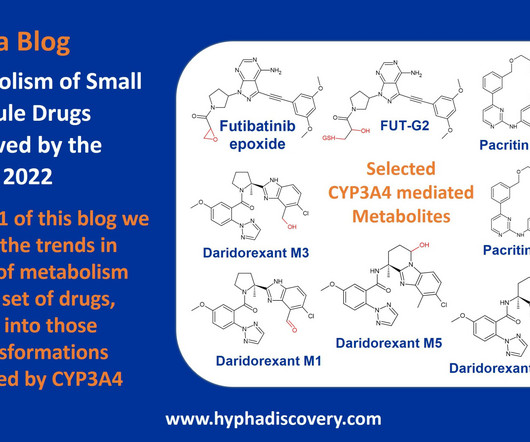
Metabolism of 2022 FDA approved small molecule drugs – Part 1 Does CYP3A4 still rule? By Julia Shanu-Wilson It won’t come as much surprise to learn that of the 17 small molecules* approved by the FDA in 2022, CYP3A4 was the major player in drug metabolism.
Metabolite Tales Blog
APRIL 4, 2023
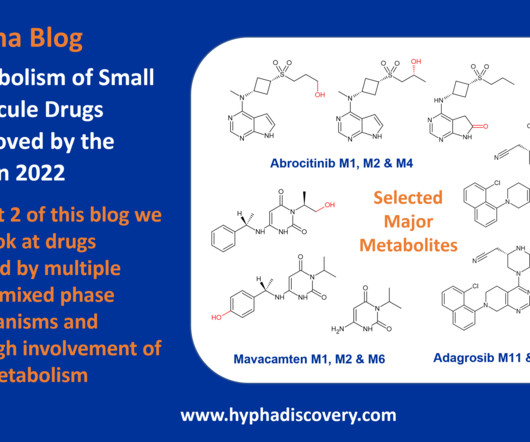
Metabolism of 2022 FDA approved small molecule drugs part 2 Mixing it Up By Julia Shanu-Wilson In Part 1 of this topic we looked at metabolism of the small molecule drugs approved by the FDA in 2022 that were mediated by CYP3A4.
Metabolite Tales Blog
APRIL 10, 2024
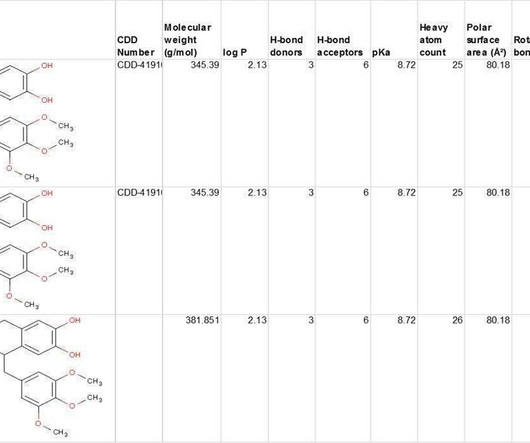
Where data was publicly available, the routes of human metabolism for each of the drugs in this subset is listed in Table 1. Metabolites found in humans are also observed in monkeys, and all metabolites were found to possess <10% of the activity of the parent drug. of the administered radiolabel in the human ADME study.

The Premier Consulting Blog
JANUARY 14, 2025
Established in 2017 under the 21st Century Cures Act, the OCE brings together multidisciplinary scientific expertise to accelerate the review and approval of drugs, biologics, and medical devices for cancer care. Provides the Oncology Dosing Toolkit. Resulting ideas will be shared with those interested in implementing them.
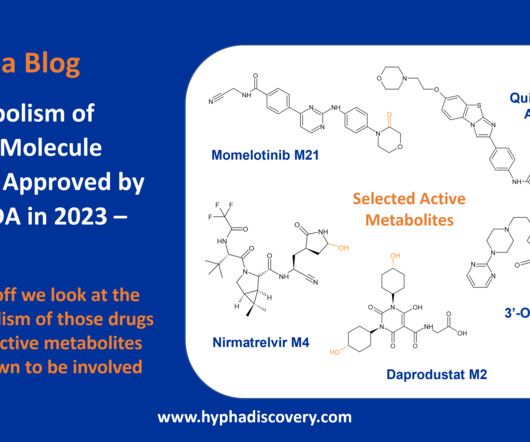
Metabolite Tales Blog
JANUARY 23, 2024
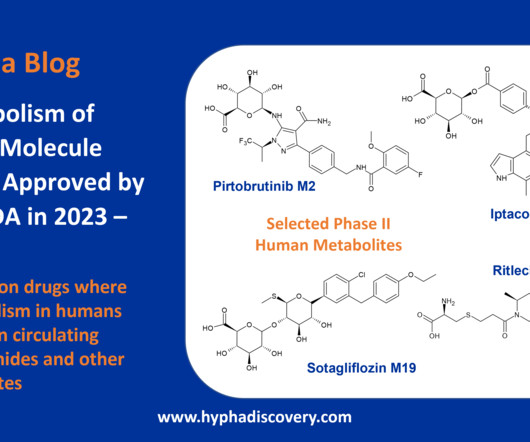
Metabolism of 2023 FDA Approved Small Molecules – PART 1 By Julia Shanu-Wilson 2023 was a fruitful year for drug approvals by the FDA, with a crop of 34 small molecules out of a total of 55 new drugs [1]. Enzymes involved include CYP1A2, CYP2C8, CYP3A4, CYP4F2, and aldehyde oxidase (AOX).

Common Sense for Drug Policy Blog
OCTOBER 29, 2024
Limited Evidence for Nalfmefene "In 2021, due to the widespread availability of high-potency synthetic opioids like fentanyl, the US FDA approved two high-dose naloxone products, an 8 mg IN spray (Kloxxado) and a 5 mg IM injectable (Zimhi). In 2023, the FDA approved a 2.7 Int J Drug Policy. doi:10.1016/j.drugpo.2024.104587

FDA Law Blog: Drug Discovery
MARCH 26, 2024
s Frank Sasinowski , James Valentine and Charles Raver are honored to have aided Italfarmaco in the development and approval of this new drug, and to be part of the effort to expand treatment options for the young men and boys living with Duchenne, and the families of those affected by Duchenne. Hyman, Phelps & McNamara, P.C.’s

FDA Law Blog: Biosimilars
JUNE 27, 2023
Valentine Named Top Lawyer Under 40; Only Food and Drug Lawyer Selected Hyman, Phelps & McNamara, P.C. (HP&M) James was only one of five life sciences attorneys selected and the only food and drug lawyer to make the list. HP&M’s James E. Valentine , as a 2023 Rising Star.

FDA Law Blog: Drug Discovery
JUNE 18, 2025
Livornese — After teasing a new rapid review pilot program for the past few weeks, on June 17, 2025, FDA officially announced the Commissioner’s National Priority Voucher (“CNPV”) program to expedite new drug and biologic (but not device or drug-device combination product) reviews. Addressing unmet public health needs.

FDA Law Blog: Drug Discovery
DECEMBER 7, 2022
Valentine — On November 22, 2022, FDA approved CSL Behring’s BLA for Hemgenix (etranacogene dezaparvovec), an AAV-based gene therapy for the treatment of adults with Hemophilia B who currently use Factor IX prophylaxis therapy, have current or historical life-threatening hemorrhage, or have repeated, serious spontaneous bleeding episodes.

FDA Law Blog: Biosimilars
OCTOBER 13, 2024
Designed to incentivize the development of drugs for pediatric rare diseases where such development may not otherwise have occurred, vouchers may be granted for drugs for serious or life-threatening rare diseases where the serious or life-threatening manifestations primarily affect individuals aged from birth to 18 years.
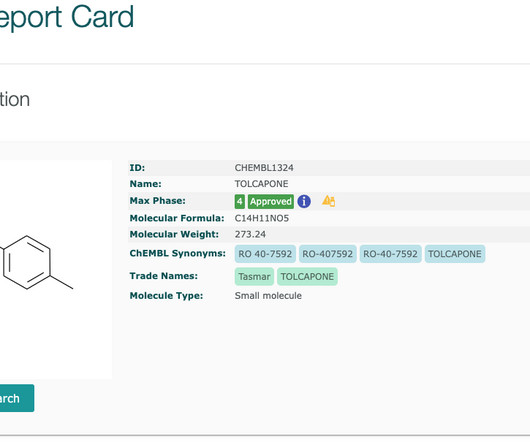
The ChEMBL-og
FEBRUARY 22, 2021
Updated drug safety information is available (as of ChEMBL 28 ) for drugs with boxed warnings and for withdrawn drugs. Boxed warnings (also know as black box warnings) are provided on medicinal product labels for FDA approved drugs if the medicinal product can cause severe or life-threatening side effects.

FDA Law Blog: Biosimilars
JANUARY 4, 2024
The American Conference Institute (“ACI”) will be hosting the go-to forum for critical updates on OTC regulation and enforcement, monograph reform, ACNU and advertising essentials… and FDA Law Blog readers can get a discount. FDA Law Blog is a conference media partner for this event.

Pharmaceutical Development Group
DECEMBER 19, 2021
Start Up and Generic Pharmaceutical Drug and Biologic Companies have high quality, affordable products that improve the quality of life for their patients. In a FDA Pre-Submission that leads to FDA Approval, more does not equal better and more does not equal relevance to a specific Pharmaceutical Drug or Biologic Product.

Pharmaceutical Development Group
JANUARY 7, 2022
Start Up and Generic Pharmaceutical Drug and Biologic Companies have high quality, affordable products and biosimilars that improve the quality of life for their patients. Seamless application of Data Standards, Data Integrity, begins no later than DMPK Protocols and all other Data filed in the Drug History File and used in the CMC.

FDA Law Blog: Biosimilars
JUNE 13, 2024
Though some drug companies have been reluctant to delist certain patents from the Orange Book, the District Court of New Jersey just ordered Teva to delist 5 of its patents that it deemed improperly listed. The Court addressed Teva’s valid argument that the Inhaler Patents are drug product patents and thus listable.

New Drug Approvals
JULY 8, 2025
1428321-10-1 Pritelivir mesylate is an antiviral drug currently under development, specifically targeting herpes simplex virus types 1 and 2 (HSV-1 and HSV-2). Pritelivir (development codes AIC316 or BAY 57-1293 ) is a direct-acting antiviral drug in development for the treatment of herpes simplex virus infections (HSV).

FDA Law Blog: Biosimilars
FEBRUARY 27, 2024
Karst — It’s been a while since we last blogged on Patent Term Extension (“PTE”) issues of interest. 156, a patent may be extended only once (even if it would be eligible for extension on more than one occasion because it applies to several FDA-approved products), and only one patent may be extended for each regulatory review period.

PerkinElmer
JANUARY 7, 2022
The pharmaceutical industry is under huge pressure to address the high attrition rates in drug development. There are many reasons that promising drug candidates are discontinued, including poor pharmacokinetics, lack of clinical efficacy, and toxicity. link] New safety concerns identified for 1 in 3 FDA-approved drugs [Internet].
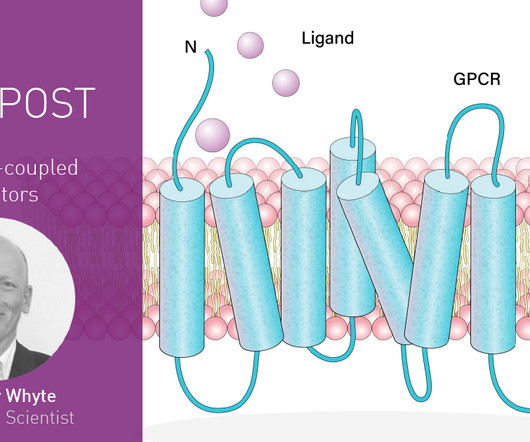
BMG Labtech
JANUARY 18, 2023
And if you need to take an FDA-approved drug, there’s around a one in three chance that it’s a drug that targets a GPCR. In the first part of this blog article, we look at what GPCRs are, some of their responsibilities in the cell, and the overall approaches used to study these signaling molecules.
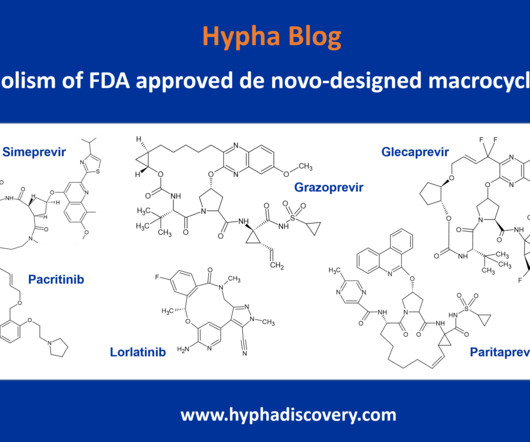
Metabolite Tales Blog
JULY 5, 2023
Metabolism of de novo-designed macrocyclic drugs approved by the FDA By Julia Shanu-Wilson To date, only 4% (67) of FDA approved drugs are macrocycles [1]. Current macrocycles in clinical use generally focus on treatment of infectious diseases, cancer and auto-immune disorders.

FDA Law Blog: Biosimilars
SEPTEMBER 19, 2023
The six-page statement explains that “Brand drug manufacturers may be harming generic competition through the improper listing of patents in the. And FDA did not provide a substantive response to 5 Requests for Advisory Opinions seeking clarification of the issue.

New Drug Approvals
JULY 10, 2025
3] Society and culture Legal status Sebetralstat was approved for medical use in the United States in July 2025. [1] 1] The US Food and Drug Administration granted the application for sebetralstat orphan drug designation. [4] PMID 36251573. ^ “Sebetralstat Orphan Drug Designations and Approvals” U.S.

Alta Sciences
APRIL 17, 2024
Five Promising Treatment Areas in Early-Phase Drug Development in 2024 aasimakopoulos Wed, 04/17/2024 - 15:52 Early-phase drug development is an ever-changing landscape, as emerging science leads to new promising areas of research for the treatment of human health issues.

New Drug Approvals
DECEMBER 19, 2022
FDA 12/1/2022, To treat adults with relapsed or refractory acute myeloid leukemia with a susceptible isocitrate dehydrogenase-1 (IDH1) mutation, Rezlidhia Olutasidenib , sold under the brand name Rezlidhia , is an anticancer medication used to treat relapsed or refractory acute myeloid leukemia. [1] 1] It is taken by mouth. [1] Hz, 1 H), 4.62−4.75
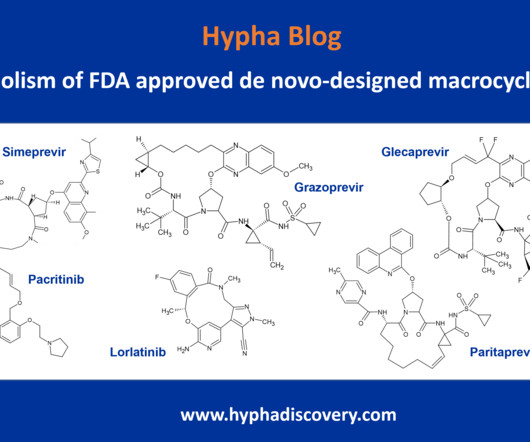
Metabolite Tales Blog
JULY 5, 2023
Metabolism of de novo-designed macrocyclic drugs approved by the FDA By Julia Shanu-Wilson To date, only 4% (67) of FDA approved drugs are macrocycles [1]. Current macrocycles in clinical use generally focus on treatment of infectious diseases, cancer and auto-immune disorders.

The Premier Consulting Blog
SEPTEMBER 11, 2024
The 505(b)(2) new drug application (NDA) pathway offers a unique opportunity for small molecule developers to bring innovative products to market more efficiently by leveraging existing data they do not own or have right of reference to. Since there was no change in drug substance, the sponsor was able to reference the DMF.

FDA Law Blog: Biosimilars
OCTOBER 1, 2024
Or consider Vanda’s challenge that FDA’s approval of a generic version of one of the company’s drugs violated the Appointments Clause of the Constitution (U.S. 2) because the FDA employees who approved the application were not “Officers of the United States.” II, § 2, cl. VANDA PHARMACEUTICALS, INC. 23-5200 (D.C.

FDA Law Blog: Biosimilars
JANUARY 18, 2024
Palmer — A new lawsuit against FDA is the latest happening in the veterinary drugs space and, by extension, FDA’s Center for Veterinary Medicine (CVM). We blogged about CVM last week and explained the increasing attention to animal health products due to the expansion of the animal and pet product market. By John W.M.

FDA Law Blog: Biosimilars
MARCH 3, 2024
Effect on FDA approvals— In cases where a biological product or drug needs to change aspects of its manufacturing processes to avoid using a covered equipment or service, will it need to file supplements with FDA for the CMC update?

Alta Sciences
FEBRUARY 8, 2024
The FDA guidance, Evaluating Drug Effects on the Ability to Operate a Motor Vehicle , indicates that testing in early-phase clinical development should emphasize sensitivity over specificity in CNS effects. View the Driving Simulation Fact Sheet for more information.

National Institute on Drug Abuse: Nora's Blog
APRIL 10, 2023
mfleming Mon, 04/10/2023 - 14:43 Nora's Blog April 13, 2023 Image Getty Images/ AleksandarGeorgiev This article originally appeared in the Milken Institute’s Power of Ideas series. Rising mortality associated with drug addiction and excessive drinking is among the major factors contributing to declining life expectancy.
Practical Cheminformatics
AUGUST 3, 2023
Most papers describing new methods for machine learning (ML) in drug discovery report some sort of benchmark comparing their algorithm and/or molecular representation with the current state of the art. Recent blog posts from Greg Landrum examined the impact of combining IC50 and Ki data from different assays.
NIH Director's Blog: Drug Discovery
MARCH 8, 2016
Now, in a new NIH-supported study, DNA barcoding helps in the development of a new method that could greatly streamline an increasingly complex and labor-intensive process: screening for drugs to combat cancer. In the next key test, the researchers exposed a mixture of 25 barcoded lung cancer cell lines to approved cancer drugs.

Molecule Blog
AUGUST 13, 2021
billion prescriptions, found that a number of prescription drugs were highly associated with longer life- and health-span in long-live populations. Specifically, we will test the drugs on human cell cultures and in fruit flies, before moving to mice in the future. In humans, no drug is currently recognized to extend lifespan.

The Premier Consulting Blog
DECEMBER 28, 2023
Clinical studies are often conducted to support a Premarket Approval (PMA) application, though some 510(k) submissions may require clinical data. In this blog post, we discuss key considerations for assessing the need for an IDE and complying with the reporting requirements under the IDE program. 2] US Food and Drug Administration.

The Premier Consulting Blog
APRIL 15, 2024
By William Salminen , Madelyn Huang, & Andrew Emanuel Without concrete guidelines, it can be confusing when determining what nonclinical studies are needed for a Pre-Investigational New Drug application (PIND) meeting. In this blog, we will go into more detail about the unwritten nonclinical requirements for the PIND meeting.

Common Sense for Drug Policy Blog
MAY 1, 2024
However, prescription drugs must be approved by the Food and Drug Administration (FDA). Although FDA has approved some drugs derived from or related to cannabis, marijuana itself is not an FDA-approved drug.
NIH Director's Blog: Drug Development
JUNE 14, 2016
Several years ago, the Food and Drug Administration (FDA) recommended that drug developers take special care to show that potential drugs to treat diabetes don’t adversely affect the cardiovascular system [1]. In fact, the evidence suggests that such drugs might even offer some protection against heart disease.

New Drug Approvals
DECEMBER 24, 2023
2] [3] [4] [5] It was approved for medical use in the United States in December 2023. [6] S2CID 250989659. ^ “Eplontersen: FDA-Approved Drugs” U.S. Food and Drug Administration (FDA). Retrieved 21 December 2023. ^ “Wainua (eplontersen) granted regulatory approval in the U.S.

New Drug Approvals
SEPTEMBER 20, 2023
1] Motixafortide was approved for medical use in the United States in September 2023. [2] 4 Similar in mechanism to the previously approved plerixafor , motixafortide is an inhibitor of C-X-C Motif Chemokine Receptor 4 (CXCR4), a protein that helps to anchor stem cells to bone marrow matrix. 1] It is given by subcutaneous injection. [1]
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content