Good things come in 3s
SugarCone Biotech
JUNE 19, 2025
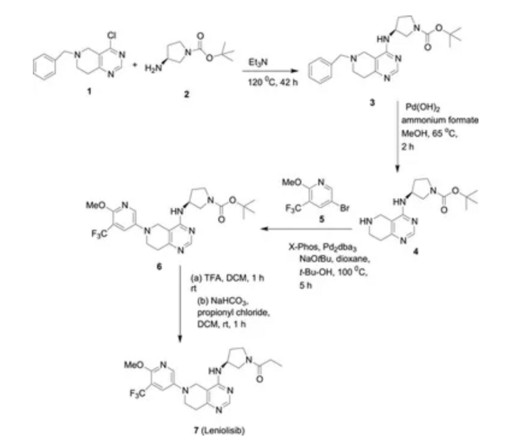
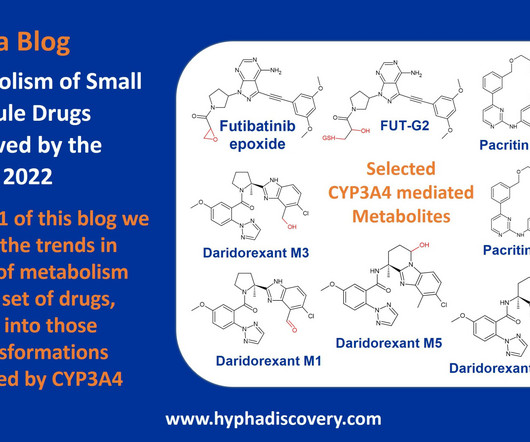
Each target and each therapeutic modality induce varying degrees of clinical efficacy, as well as causing toxicities. Finally, while there are clear front runners among the pharmaceutical companies developing targeted therapeutics for these antigens there are also emerging biotechs aggressively pursuing these targets.















































Let's personalize your content