The rising impact of biomarkers in early clinical development
Drug Target Review
APRIL 7, 2025
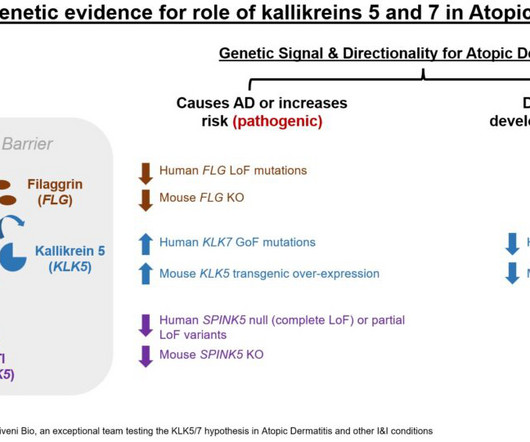
As our understanding of the underlying biology of disease grows more sophisticated, emerging therapies operate on increasingly complex biopathological systems and mechanisms. Monitoring biomarkers can help assess changes in a disease, its level of expression, or the extent of its progression.






































Let's personalize your content