How AI will reshape pharma by 2025
Drug Target Review
DECEMBER 20, 2024
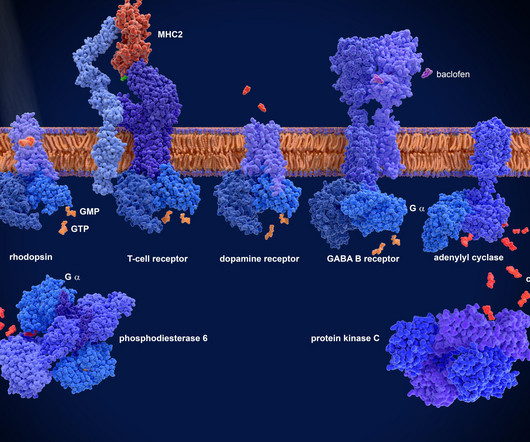
Their work focuses on creating ‘digital twin generators’ – AI-driven models that predict how a patients disease may progress over time. These digital twins allow pharmaceutical companies to design clinical trials with fewer participants, while still providing reliable evidence to assess a drugs effectiveness.


















Let's personalize your content