GSK to buy liver disease drug for $1.2B
BioPharma Drive: Drug Pricing
MAY 14, 2025
The new acquisition from Boston Pharmaceuticals adds to a wave of dealmaking undertaken by GSK to build its liver disease franchise.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

BioPharma Drive: Drug Pricing
MAY 14, 2025
The new acquisition from Boston Pharmaceuticals adds to a wave of dealmaking undertaken by GSK to build its liver disease franchise.
Drug Target Review
JUNE 9, 2025
The landscape of genetic medicine is undergoing a profound transformation, driven by innovative approaches that challenge the traditional, disease-specific paradigms. My transition to biotech and rare diseases was deeply personal – my son was diagnosed with Duchenne muscular dystrophy in 2020. “My
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Drug Target Review
DECEMBER 20, 2024
Artificial intelligence (AI) has revolutionised many industries, yet its adoption in pharmaceutical drug development has been notably slower. Looking ahead, 2025 could represent a major turning point for the pharmaceutical sector.
Drug Target Review
JUNE 16, 2025
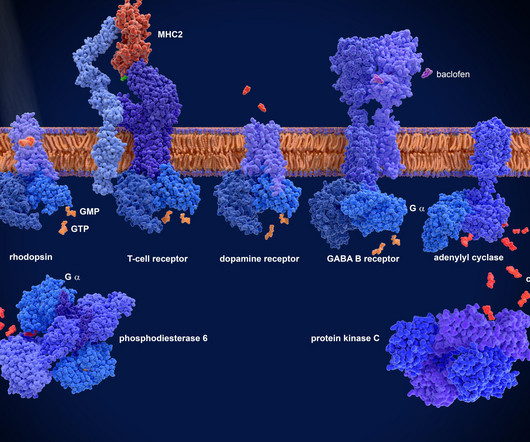
Targeting GPCRs to fight inflammatory diseases GPCR-targeting drugs are well known therapies for a range of disease types, including cardio-metabolic, central nervous system and endocrinological disorders. This makes conventional approaches, which attempt to target the orthostatic binding sites, less effective.

Drug Target Review
JULY 1, 2025
Placebo arms, in particular, create ethical and logistical hurdles, especially in areas like rare disease and oncology. They are patient-specific outcome predictions, generated using disease-specific machine-learning models trained on large, longitudinal clinical datasets. Digital twins offer a way forward.

Broad Institute
JUNE 11, 2025
First launched in 2015 and renewed in 2020, the program began as a recognition that while a majority of cardiovascular disease can be associated with lifestyle factors such as tobacco consumption, diet, and level of physical activity, genomics can influence an individual’s predisposition to cardiovascular disease, age of onset, and severity.

Broad Institute
JULY 29, 2025
It is already used throughout the pharmaceutical industry to discover the causes of and treatments for disease, but recently the team recognized the potential of Cell Painting, along with transcriptomic and proteomic methods, for toxicity testing.

Crown Bioscience
AUGUST 5, 2025
The shift from traditional 2D cell lines to 3D organoids provides a more relevant context for understanding diseases and testing treatments. For decision-makers in the pharmaceutical and biotech industries, organoids represent a key innovation extending beyond existing in vitro models.

Crown Bioscience
AUGUST 5, 2025
The shift from traditional 2D cell lines to 3D organoids provides a more relevant context for understanding diseases and testing treatments. For decision-makers in the pharmaceutical and biotech industries, organoids represent a key innovation extending beyond existing in vitro models.
Drug Target Review
OCTOBER 30, 2024
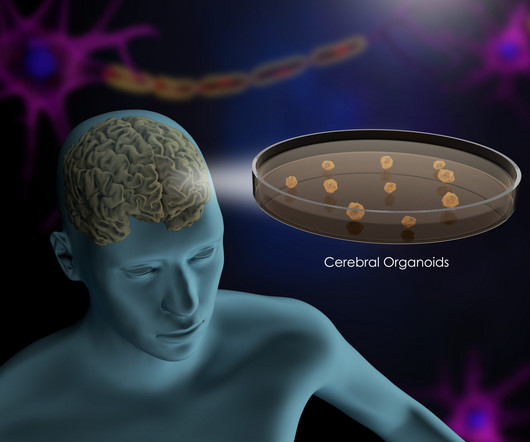
Imagine being able to create an in vitro replica of a diseased organ to study the molecular mechanism underlying the illness. Now take a step further: envision testing drugs in these organoids to identify the ones that can treat disease safely and effectively without needing to run expensive clinical trials first.
Drug Target Review
MARCH 3, 2025
The search for effective treatments for neurodegenerative diseases like Parkinson’s disease has long been hindered by the brain’s complexity and the absence of adequate models for drug discovery. “For diseases like Parkinson’s, it’s more than sufficient,” he explains.
Drug Patent Watch
DECEMBER 12, 2024
This approach not only helps maintain market exclusivity but also ensures a steady revenue stream for pharmaceutical companies. Understanding the Pharmaceutical Market Dynamics The pharmaceutical industry is a complex ecosystem where branded drugs and generics coexist, each playing a vital role in patient care and market dynamics.

Drug Target Review
JULY 21, 2025
Disorders such as Alzheimer’s disease , primary glioblastoma and amyotrophic lateral sclerosis (ALS) all affect the CNS and are considered fatal, while more common conditions, including depression , strokes and epilepsy , require long-term treatments. Hundreds of life-altering conditions attack the central nervous system (CNS).

The Pharma Data
JUNE 25, 2025
Novartis Finalizes Acquisition of Regulus Therapeutics, Strengthening Its Renal Disease Portfolio with Promising ADPKD Therapy Novartis AG, a leading global pharmaceutical company, has officially completed its acquisition of Regulus Therapeutics Inc. , a biotechnology firm known for its expertise in microRNA-targeting therapies.

Drug Target Review
JULY 3, 2025
It is becoming increasingly evident that generative artificial intelligence (GenAI) is a resourceful tool for helping pharmaceutical companies reduce manual tasks required by clinical trials. This is especially relevant with today’s heavier focus on enhancing personalised medicine via broader emerging scientific findings.

Drug Target Review
AUGUST 6, 2025
A validated target in cardiovascular disease, new research from Esperion Therapeutics suggests that ACLY could also play a role in liver disease – specifically, primary sclerosing cholangitis (PSC). The approach could offer a new angle on a disease that has thus far resisted most conventional drug development strategies.

Drug Target Review
JUNE 11, 2025
NGS has revolutionised genomic analysis, enabling the identification of disease-related genetic variants. Multimodal language models Generative AI models (GenAI) in the pharmaceutical field have reached the highest level of attention with the Nobel Prizes to Demis Hassabis and John Jumper for AlphaFold, which can predict protein structures.

Drug Patent Watch
DECEMBER 9, 2024
In the vast realm of pharmaceutical research and development, there’s a fascinating intersection between ancient wisdom and modern science. “Pharmacognosy is the bridge between traditional medicine and modern pharmaceutical science, offering a treasure trove of potential new drugs waiting to be discovered.”

The Pharma Data
JUNE 25, 2025
The goal is to boost SMN protein production more effectively and offer a more convenient, once-a-year dosing schedule , thereby improving long-term disease management and patient compliance. These reductions were sustained through one year , highlighting salanersen’s potential to meaningfully slow disease progression. Source link

Drug Target Review
JUNE 5, 2025
Despite recent advances in gene therapy for sickle cell disease (SCD) , automated red blood cell exchange (aRBCX) remains a cornerstone therapy that plays a vital yet underutilised role in managing complications and enhancing quality of life for millions living with this devastating condition worldwide.

Drug Target Review
DECEMBER 11, 2024
This shift in focus is especially critical in toxicology, where accurate target analysis plays a vital role in identifying toxic effects and ensuring patient safety, particularly as the field transitions from traditional drugs to the promising realm of biotherapeutics, especially for rare diseases.

The Pharma Data
JUNE 9, 2025
This new chapter in the strategic partnership between WuXi Biologics and VISEN Pharmaceuticals underscores a shared commitment to innovation, localization, and improved patient care. VISEN Pharmaceuticals expects to obtain regulatory approval for lonapegsomatropin in China by 2025. Chen said. “By

The Pharma Data
JUNE 16, 2025
Teva and Fosun Pharma Forge Strategic Partnership to Develop and Commercialize Innovative Anti-PD1-IL2 Therapy (TEV-56278) in Immuno-Oncology Teva Pharmaceutical Industries Ltd. and Shanghai Fosun Pharmaceutical (Group) Co., About Fosun Pharma (Shanghai Fosun Pharmaceutical (Group) Co.,
PPD
OCTOBER 31, 2024
As the pharmaceutical industry continues to evolve, drug developers encounter new challenges and opportunities in their pursuit of innovation. This issue is particularly pronounced for rare diseases and trials requiring diverse patient populations.

The Pharma Data
AUGUST 1, 2025
Arrowhead’s Subsidiary Visirna Transfers Greater China Rights to Plozasiran to Sanofi in Strategic $395 Million Deal Amid Regulatory Milestone in Hypertriglyceridemia In a move poised to reshape the therapeutic landscape for patients with severe lipid disorders in China, Arrowhead Pharmaceuticals , Inc. President and CEO of Arrowhead. “We

BioPharma Drive: Drug Pricing
JULY 8, 2025
Love Employee via Getty Images For decades now, scientists have been experimenting with a new way to target and destroy proteins linked to disease. It may also be a more efficient way to shut down known disease targets. Kymera Therapeutics is working on protein degraders for a wide range of immune diseases, and some cancers.

The Pharma Data
AUGUST 1, 2025
Accelerating Innovation in Ocular Drug Development The initial focus of the partnership will be on ocular diseases, a complex and underserved therapeutic area in which preclinical models often fall short of predicting human responses. Ocular indications are an ideal starting point for our collaboration,” added Valente.

The Pharma Data
JUNE 9, 2025
According to Dr. Kelvin Tan , Chief Medical Affairs Officer at Jazz Pharmaceuticals, the data add to the growing body of evidence supporting sodium reduction as a modifiable risk factor. The new late-breaking data presented by Jazz Pharmaceuticals at SLEEP 2025 solidify Xywav’s position as a cornerstone treatment in narcolepsy management.

Drug Patent Watch
JANUARY 14, 2025
In the fast-paced world of pharmaceuticals, negotiations play a pivotal role in shaping the industry’s landscape. Let’s dive into the intricacies of pharmaceutical negotiations, exploring valuable lessons, current trends, and strategies for success.

The Pharma Data
JUNE 18, 2025
While localized prostate cancer boasts a five-year survival rate above 90% , that figure drops precipitously to approximately 31% for metastatic disease. It will enroll patients with mCRPC who have experienced disease progression during or after ARPI therapy.

The Premier Consulting Blog
NOVEMBER 6, 2024
Inhaled combination products (ICP) have emerged as a significant advancement in the treatment of respiratory diseases such as asthma, chronic obstructive pulmonary disease (COPD), and other pulmonary conditions. The goal of ICPs is to improve the quality of life for patients suffering from chronic respiratory diseases.

Drug Patent Watch
MAY 14, 2025
"Reviving Old Medicines: How AI-Powered Repurposing is Revolutionizing the Pharmaceutical Industry The pharmaceutical industry is on the cusp of a revolution, driven by the power of computational drug repurposing.

Conversations in Drug Development Trends
OCTOBER 29, 2024
By: Juliane Mills, Senior Director, Therapeutic Strategy Lead, Rare Disease The rise of patient-led clinical research, particularly in rare disease, represents a significant shift in the clinical trial landscape. Why Is There an Increase in Patient-Led Rare Disease Research?

The Pharma Data
JUNE 24, 2025
Biologic drugs, including monoclonal antibodies, have become essential in the treatment of a wide array of conditions, from autoimmune diseases to various cancers. To date, more than 160 monoclonal antibody therapies targeting nearly 100 disease-related proteins have received regulatory approval globally.
Drug Target Review
JUNE 16, 2025
Mass spectrometry imaging (MSI) enables the direct detection and quantitation of active pharmaceutical ingredients (APIs) and metabolites within tissue sections, making it widely regarded as a promising technique in the field of pharmacology and toxicology. Bioanalysis 2019, 11 (11), 1099–1116. Cheng S-H, Groseclose MR, Mininger C, et al.

The Pharma Data
JUNE 18, 2025
Alnylam Elevates Dr. Pushkal Garg to Lead Unified Research and Development Organization, Signaling Strategic Expansion into Next Phase of RNAi Therapeutics Innovation Alnylam Pharmaceuticals , a global leader in RNA interference (RNAi) therapeutics, has announced the promotion of Pushkal Garg, M.D.,

Drug Target Review
JULY 31, 2025
The scalability and cost-effectiveness of recombinant production have also democratised access to these improved antibodies, allowing academic labs and small biotech startups to leverage the same advanced tools once limited to large pharmaceutical companies.

The Pharma Data
JUNE 15, 2025
Sanofi and Regeneron’s Dupixent Shows Superiority over Xolair in First-Ever Head-to-Head Phase 4 Study in Patients with Severe Chronic Rhinosinusitis with Nasal Polyps and Co-existing Asthma Sanofi and Regeneron Pharmaceuticals, Inc. points greater in reduction with Dupixent (p < 0.00011). The asthma control score improved by 0.48

The Pharma Data
JUNE 6, 2025
Otsuka Unveils Promising Phase 3 Results for Sibeprenlimab in IgA Nephropathy, Marking Significant Proteinuria Reduction and Advancing a Novel APRIL-Targeted Therapy Otsuka Pharmaceutical Development & Commercialization, Inc., in collaboration with Otsuka Pharmaceutical Co.,

Reprocell
AUGUST 11, 2025
When examining the existing landscape of pharmaceutical interventions for inflammatory bowel disease (IBD), the inherent limitations of current pre-clinical models and how it is hampering the translation of research findings into new therapeutic approaches are readily apparent.

The Pharma Data
JUNE 11, 2025
Ionis Begins Pivotal Phase 3 REVEAL Study of ION582 in Angelman Syndrome, Dosing First Patient in Global Trial Ionis Pharmaceuticals , Inc. This trial will build on previous positive data from the earlier Phase 1/2 HALOS study and is designed to rigorously assess the potential of ION582 as a disease-modifying therapy. “We Kordasiewicz.

The Pharma Data
JUNE 22, 2025
Engineered by Chugai Pharmaceutical Co., Roche’s continued investment in this space underscores a larger commitment to reducing the burden of disease on individuals and healthcare systems alike. Importantly, the therapy demonstrated a favorable safety profile, with no thromboembolic events reported so far.

Broad Institute
NOVEMBER 25, 2024
Ladders to Cures (L2C) Accelerator By Maria Nemchuk November 25, 2024 Breadcrumb Home Ladders to Cures (L2C) Accelerator The Ladders to Cures (L2C) Accelerator aims to catalyze progress across the research ecosystem and accelerates advances leading to treatments and cures for patients with rare genetic diseases.
Drug Target Review
DECEMBER 9, 2024
Its applications include studying disease pathology to develop targeted therapeutics, visualising enzymes that can decompose plastics, and engineering solutions to antibiotic resistance, among many others. David Baker is listed as an inventor on many patents, focused on compositions and methods for treating particular diseases.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content