The future of CNS drug development: signs of real progress
Drug Target Review
JULY 21, 2025
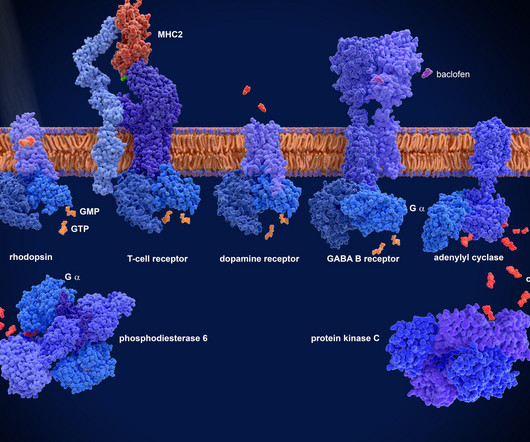
2 A raft of emerging therapeutic modalities sits at the centre of this boom, spanning advanced biologics, engineered platforms and next-generation small molecules. However, the advanced nature of the drugs being developed has brought new challenges. The global market for CNS therapeutics was worth an estimated $144.3








































Let's personalize your content