Researchers restore antibiotic effect in the event of resistance
Science Daily: Pharmacology News
MAY 7, 2025
Bacterial resistance negates the effect of antibiotics in the treatment of infection.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Science Daily: Pharmacology News
MAY 7, 2025
Bacterial resistance negates the effect of antibiotics in the treatment of infection.

Science Daily: Pharmacology News
JANUARY 22, 2025
The trial was stopped early by the recommendation of the Data Monitoring Committee due to an overwhelming reduction in bleeding compared to standard-of-care treatment.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

ASPET
SEPTEMBER 16, 2024
Post-traumatic stress disorder (PTSD) is a debilitating psychiatric condition that develops following exposure to a traumatic event. In most patients, currently available pharmacological and psychological treatments are insufficient to alleviate the array of symptoms associated with the disorder.
Conversations in Drug Development Trends
APRIL 29, 2025
The day inspires multiple events, such as fundraisers and scientific symposia, to bring together all the critical stakeholders who tirelessly work to make life better for those affected by rare diseases. In an incredible feat of collaboration, they were able to rapidly put together a new event called Rally for Rare.

Drug Target Review
MARCH 17, 2025
Neuropsychiatric treatment is on the verge of a major transformation. Historically, treatment options have been limited, with patients relying on daily medications that have minimal efficacy and troublesome side effects. “We call this phenomenon ‘event-driven pharmacology.”

Drug Target Review
JUNE 5, 2025
Dr Aaron Haubner, Senior Manager of North America Medical Affairs and Market Access at Terumo Blood and Cell Technologies , reveals that while promising new treatments emerge, urgent partnerships are needed to ensure this essential blood therapy reaches the patients who need it most. There was a predicted 21.5
Drug Target Review
DECEMBER 23, 2024
While these therapies hold great promise for improving cancer treatment outcomes, their development presents significant challenges, especially in achieving the optimal balance between efficacy and safety. Properly managing these toxicities is crucial to enhancing the safety and therapeutic effectiveness of ADC treatments.

ASPET
SEPTEMBER 29, 2023
Recent studies have yielded controversial results on the long-term effects of statin treatment on the risk of cardiovascular (CV) events. The incidence of first CV events was 6.0% Taken together, our data demonstrate that a 7-year stable control of LDL-C induces a forty percent reduction in the incidence of CV events.

ASPET
SEPTEMBER 20, 2024
Post-traumatic stress disorder (PTSD) is caused by exposure to a traumatic or stressful event. Current pharmacological treatments for PTSD are insufficient, with fewer than 30% of patients reporting symptom remission. Treatments began 4 hours after FC. The OFT was conducted one day before the last FC re-exposure.

Drug Target Review
APRIL 7, 2025
Haemoglobin A1c (HbA1c) is a validated surrogate endpoint for the reduction of microvascular complications associated with diabetes mellitus; reduced HIV-RNA levels serve as an endpoint for HIV disease control; and a reduction in low-density lipoprotein (LDL) cholesterol is used as an endpoint indicating lower likelihood of cardiovascular events.

SCIENMAG: Medicine & Health
JUNE 24, 2023
About The Study: The findings of this study suggest that treatment with bempedoic acid in high-risk primary prevention patients unable to tolerate recommended doses of statins has the potential to reduce major adverse cardiovascular events. Authors: Steven E. Nissen, M.D., of the Cleveland Clinic, is the corresponding author.

Chemical Biology and Drug Design
FEBRUARY 13, 2024
Some researchers proposed that the symptoms involved in AD (loss of memory, cognitive impairment, and amnesia) are the main event linked to the cholinergic neurotransmitter (acetylcholine). This review focuses on the last 10 year's literature search on huprine and its analogues for the treatment of AD.
New Drug Approvals
APRIL 11, 2025
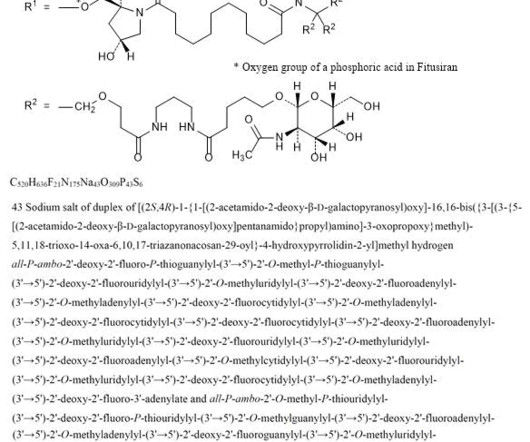
1] [2] Adverse effects The US Food and Drug Administration prescription label for fitusiran contains a boxed warning for thrombotic events (blood clotting) and gallbladder disease (with some recipients requiring gallbladder removal). [2] Fitusiran 1711.0g/mol, 1] It is an antithrombin-directed small interfering ribonucleic acid. [1]
PLOS: DNA Science
MARCH 28, 2024
FDA classifies it as a “nonsteroidal treatment” – not a gene therapy, but it affects gene expression. A gene-based treatment would have to alter many cells to exert a noticeable effect. In 2023, two gene-based treatments became available. Muscle makes up about 40 percent of body weight. million DNA bases.

Broad Institute
FEBRUARY 25, 2025
While more than 8,000 genes are known to drive these diseases, fewer than 500 have an available treatment. The Ladders to Cures Scientific Accelerator is committed to uncovering the genetic roots of rare diseases and to using those insights to develop new treatments.

The Pharma Data
JUNE 13, 2025
Currently, treatment options for patients with relapsed or refractory disease remain limited, and prognosis is poor. Standard single-target CD19-directed CAR T-cell therapies typically enable long-term remissions in roughly 40% of patients, emphasizing a significant unmet need for new and more effective treatment strategies.

The Pharma Data
JUNE 18, 2025
The double-blind treatment period lasted 24 weeks, during which both efficacy and adverse events (AEs) were closely monitored. of patients in the atogepant arm discontinued treatment because of AEs, versus a substantially higher 29.6% Specifically, only 12.1% among those taking topiramate. to 0.59; p <0.0001).
Broad Institute
JULY 11, 2024
Targeted drug treatment leads tumor cells to imitate viral infection By Ari Navetta July 11, 2024 Breadcrumb Home Targeted drug treatment leads tumor cells to imitate viral infection Exploiting "viral mimicry," mIDH1 inhibitors trick tumors into thinking they are infected with a virus.

The Pharma Data
JUNE 15, 2025
The data, which encompass up to 3 years of follow-up after a single infusion of the treatment, were shared in an oral presentation at the European Academy of Allergy and Clinical Immunology (EAACI) Congress 2025, held from June 13–16 in Glasgow, United Kingdom. President and Chief Executive Officer of Intellia Therapeutics.

The Pharma Data
JUNE 12, 2025
The data were revealed during an oral presentation (S137) at the 2025 European Hematology Association (EHA) Congress in Vienna, reflecting ongoing progress in developing new treatment options for patients battling this aggressive hematologic malignancy.

The Pharma Data
JUNE 15, 2025
This is crucial information for guiding both patients and physicians in their treatment decisions.” All patients received their respective biologic therapy in addition to standard background treatment with mometasone furoate nasal spray (MFNS). Serious adverse events were somewhat lower in Dupixent (2%) than in omalizumab (4%).

The Pharma Data
JUNE 16, 2025
Currently, the medial survival for patients with MZL after multiple lines of treatment falls within the range of 3–5 years, reflecting a significant and persistent need for more effective therapeutic options. Neurologic events (NE) of any grade were documented in 33% of patients, with Grade 3 NEs in 4% and no Grade 4 or 5 NEs.

The Pharma Data
JUNE 11, 2025
The investigational therapy, an Fc-free anti-CD40L monoclonal antibody, is being developed for the treatment of systemic lupus erythematosus (SLE), a chronic autoimmune disease with limited treatment options and significant unmet need. Treatment-Emergent Adverse Events (TEAEs) : Occurred in 82.6% in the SOC group.

The Pharma Data
JUNE 15, 2025
According to the data, the combination demonstrated a high ORR and a strong depth of response in a patient population with limited treatment options and poor outcomes under standard care. were penta-drug refractory, having been exposed to additional lines of treatment. Among these: 84.4% had previously received a bispecific antibody.

Broad Institute
NOVEMBER 25, 2024
Ladders to Cures (L2C) Accelerator By Maria Nemchuk November 25, 2024 Breadcrumb Home Ladders to Cures (L2C) Accelerator The Ladders to Cures (L2C) Accelerator aims to catalyze progress across the research ecosystem and accelerates advances leading to treatments and cures for patients with rare genetic diseases. Visit broad.io/L2C2023

The Pharma Data
JUNE 9, 2025
The COACH study is the first clinical investigation to assess the combined effects of two once-weekly treatments—TransCon® CNP (navepegritide) and TransCon® hGH (lonapegsomatropin)—in children with achondroplasia. TransCon CNP as a monotherapy has demonstrated the potential to transform the treatment of achondroplasia,” Dr. Shu stated.

FDA Law Blog: Biosimilars
APRIL 13, 2025
LYTGOBI was granted accelerated approval for the treatment of adult patients with previously treated, unresectable, locally advanced, or metastatic intrahepatic cholangiocarcinoma (iCCA) with FGFR2 gene fusions or rearrangements. Join us as we break down what went wrong this time. What Happened? The problem? As a single-arm trial (i.e.,

The Pharma Data
JUNE 12, 2025
The results come from a open-label, proof-of-concept, Phase 2 study (NCT04520451) and highlight rilzabrutinib’s potential as a disease-changing treatment option for a condition that currently has limited and non-specific treatment options and involves substantial patient suffering due to its chronic and progressive course.

The Pharma Data
JUNE 9, 2025
Food and Drug Administration (FDA) for the treatment of cataplexy or excessive daytime sleepiness (EDS) in patients aged seven years and older with narcolepsy. of participants reported treatment-emergent adverse events (TEAEs), all of which were mild or moderate and consistent with previous safety data on Xywav.

The Pharma Data
JUNE 11, 2025
Achieving the Primary Endpoint: Sotyktu Demonstrates Superiority Over Placebo The POETYK PsA-1 trial, officially designated as study IM011-054, enrolled patients with active PsA who had not received prior treatment with biologic disease-modifying antirheumatic drugs (bDMARDs).
KIF1A
JULY 15, 2023
When the brain’s electrical activity changes enough to cause noticeable changes in behavior, we call that event a seizure. By identifying short and subtle changes in movements, we may be able to characterize epileptic events with more precision. From Gschwind et al. 2023, Neuron.
DrugBank
OCTOBER 17, 2024
Bayesian adaptive randomization , for example, can dynamically allocate patients to different treatment arms based on accumulating data, ensuring that more patients receive the most promising treatment. Machine learning algorithms can be trained on historical data to identify patterns and anomalies indicating potential safety concerns.

PPD
AUGUST 7, 2024
Clinical trials are the backbone of medical research, enabling the development of new treatments and therapies that can improve patient outcomes. Adverse Event Reporting and Safety Monitoring: During a clinical trial, participants may experience adverse events that may be related or unrelated to the study drug.
Drug Target Review
AUGUST 6, 2024
Autoimmune diseases : Rheumatoid Arthritis : ADCs targeting specific immune cells or inflammatory mediators can provide more precise treatment options with potentially fewer side effects. Obesity : By targeting adipose tissue or specific metabolic pathways, ADCs could offer new treatments for obesity and related metabolic disorders.

ASPET
JUNE 30, 2023
Myeloproliferative Neoplasms (MPNs) are hematological malignancies that result from acquired driver mutations in hematopoietic stem cells (HSCs), causing overproduction of blood cells and an increased risk of thrombo-hemorrhagic events. This study aims to determine a personalized treatment strategy.

The Pharma Data
JUNE 11, 2025
argenx Unveils Positive Phase 2 Results for Efgartigimod in Myositis and Sjogren’s Disease at EULAR 2025 argenx SE a global immunology company dedicated to advancing treatments for severe autoimmune diseases, presented encouraging new clinical data at the 2025 European Congress of Rheumatology (EULAR), held June 11–14 in Barcelona, Spain.

Chemical Biology and Drug Design
MAY 11, 2023
Abstract Parkinson's disease is among the most common forms of neurodegenerative illness, with present treatment being primarily symptomatic and frequently coming with substantial adverse effects. Therefore, this compound is considered a safe and effective therapeutic choice for neurodegenerative illnesses like Parkinson's disease.

SCIENMAG: Medicine & Health
JANUARY 16, 2024
Scaling drugs to a patient’s weight prevents adverse events from overtreatment and treatment failure due to underdosing. Knowing a patient’s weight is necessary for many weight-based medications such as thrombolytics, anticoagulants and numerous cardiovascular medications. Credit: Alex Dolce, Florida Atlantic University […]

Elrig
MARCH 25, 2025
Prof Rory Johnson, Associate Professor, University College Dublin, and Dr Shalini Andersson, Vice President Nucleic Acid Therapeutics, AstraZeneca will lead this years event focussed on drugging the undruggable.
Broad Institute
JUNE 28, 2023
The findings, published today in Nature , could lead to new ways of guiding cancer treatment or developing targeted drugs. We began to think that these shorter events could give us signals about whether cancer cells were selecting for certain chromosome changes.”

The Pharma Data
JUNE 6, 2025
This outcome suggests sibeprenlimab’s potential to not only delay disease advancement but also improve long-term renal outcomes, addressing a critical unmet need for patients with IgAN, many of whom currently face limited treatment options. Treatment-emergent adverse events (TEAEs) occurred in 76.3% in the placebo group.

FDA Law Blog: Drug Discovery
JANUARY 30, 2025
This target completion date is informed by the natural history of the disease, availability of alternative treatment, anticipated recruitment timeline, and the projected timeline for efficacy analysis(es); 2) the sponsors progress and plans for postapproval conduct of the trial provide sufficient assurance to expect timely completion of the trial.

The Pharma Data
MAY 16, 2022
Food and Drug Administration (FDA) has lifted the clinical hold placed on the company’s Investigational New Drug Application (IND) to evaluate injectable lenacapavir for HIV treatment and HIV pre-exposure prophylaxis (PrEP). There is no cure for HIV or AIDS. About Lenacapavir.
SCIENMAG: Medicine & Health
NOVEMBER 12, 2023
Low levels of Vitamin D have been shown to be associated with a higher risk of having a cardiac event, like a heart attack or stroke. For this reason, treatment by Vitamin D pills or injections are being investigated as a possible preventative method in these patients. Credit: Intermountain Health Low levels of Vitamin D […]
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content