Inavolisib
New Drug Approvals
APRIL 25, 2025
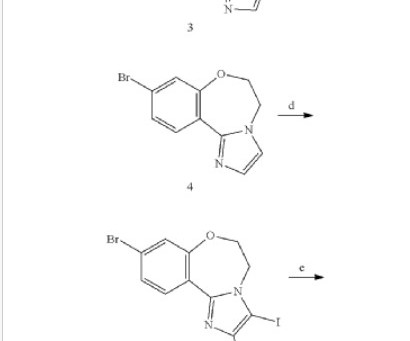
3] Inavolisib was approved for medical use in the United States in October 2024. [3] Members of the PI3K family regulate cellular processes such as cell growth and proliferation, survival, remodelling, and intracellular transport of organelles. [15] Food and Drug Administration (FDA). 3 November 2006. Retrieved 17 April 2025.















































Let's personalize your content