Analyzing Oxygen Atom Distribution in FDA‐Approved Drugs to Enhance Drug Discovery Strategies
Chemical Biology and Drug Design
FEBRUARY 6, 2025
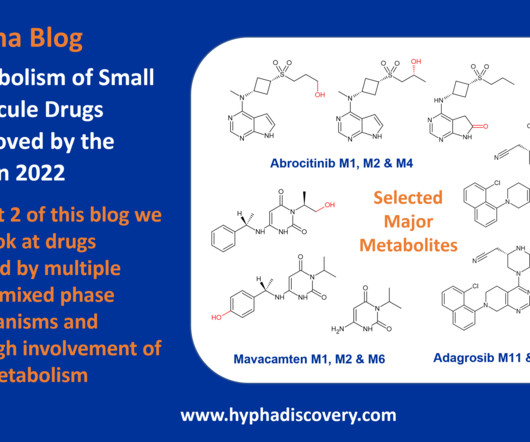
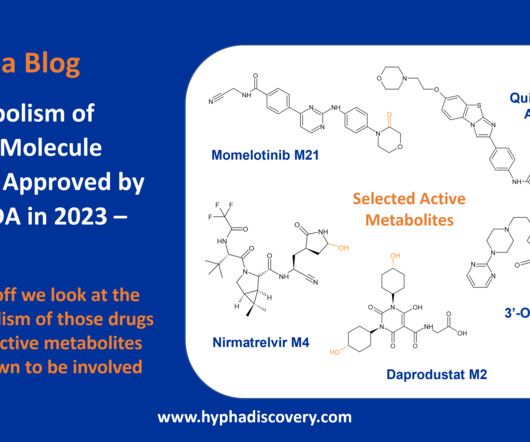
This work presents a comprehensive analysis of oxygen atoms in approved drugs, aiming to streamline drug design and discovery efforts. The study examines the frequency, distribution, prevalence, and diversity of oxygen atoms in a dataset of 2049 small molecules approved by the FDA and other agencies.



































Let's personalize your content