Teva and Fosun Partner to Develop Novel Anti-PD1-IL2 Immunotherapy
The Pharma Data
JUNE 16, 2025
Teva and Fosun Pharma Forge Strategic Partnership to Develop and Commercialize Innovative Anti-PD1-IL2 Therapy (TEV-56278) in Immuno-Oncology Teva Pharmaceutical Industries Ltd. TEV-56278 is a first-in-class anti-PD1-IL2 ATTENUKINE therapy designed by Teva’s internal team of innovative scientists.


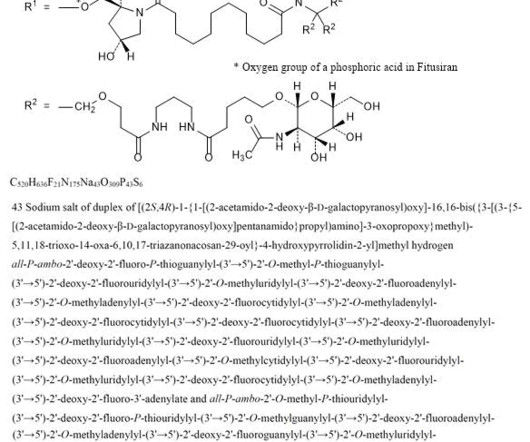









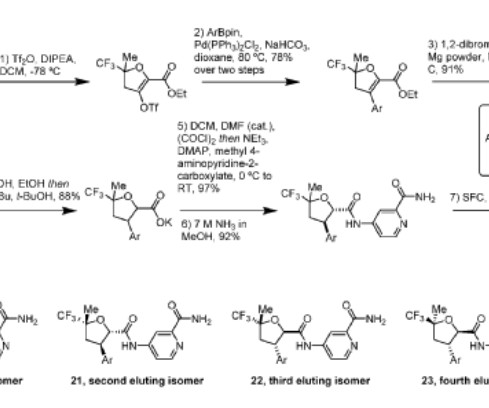

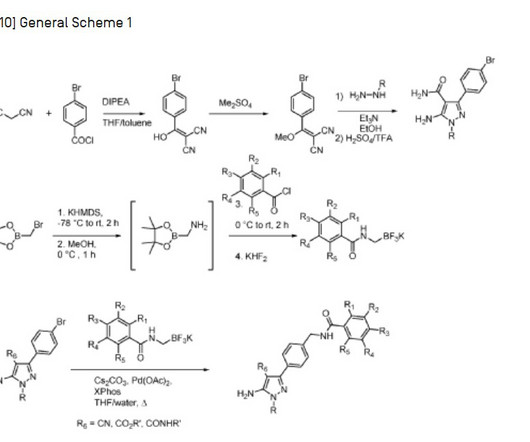


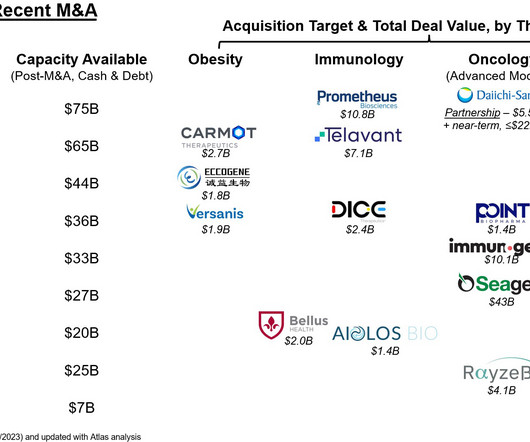


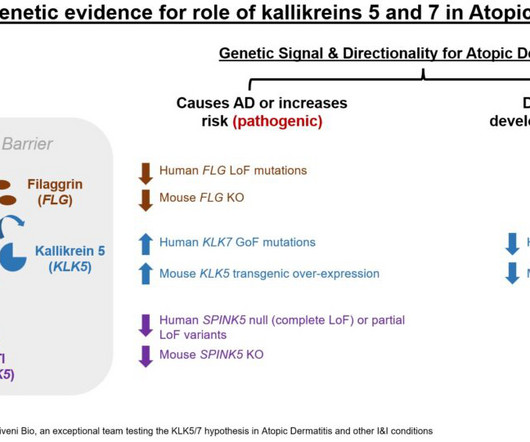


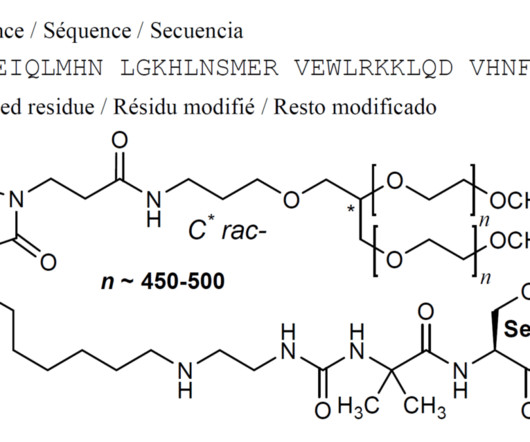
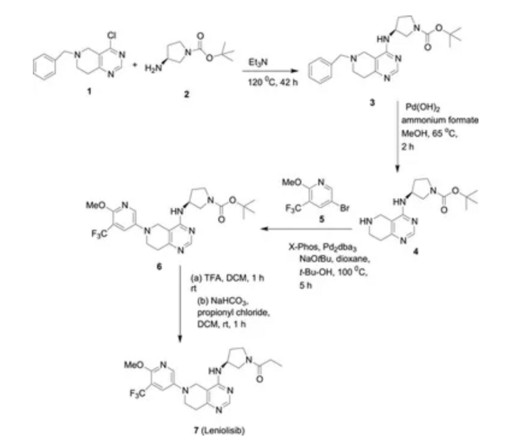






















Let's personalize your content