The Uncertain Origins of Aspirin
Codon
JULY 14, 2025
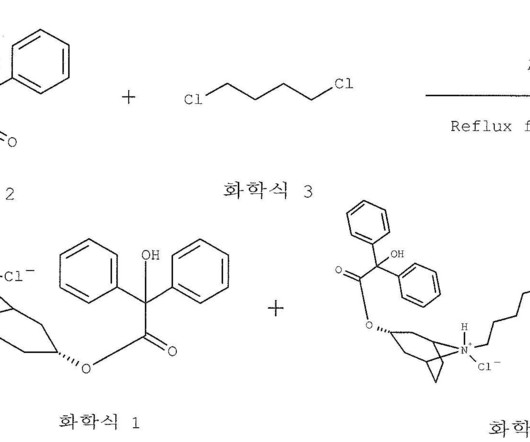
Ancient History: Antiquity to 1763 AD When I was in medical school in 2007, I learned that willow bark tea has been used for thousands of years to treat pain and fever. NSAIDs may likewise play some as yet undetermined role in neurodegenerative diseases, diabetes, and cancer disease therapy. Join Asimov Press.

















Let's personalize your content