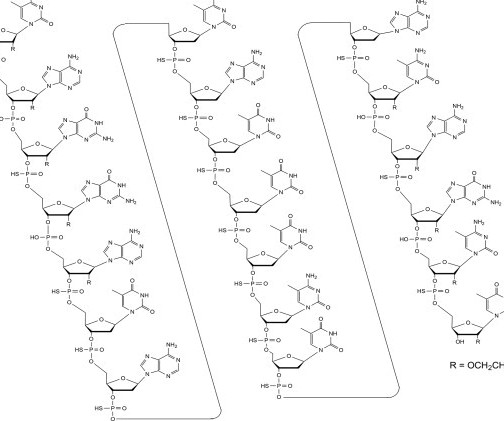
Tofersen
New Drug Approvals
MAY 17, 2025
Jump up to: a b c d e f g h i j k l “FDA approves treatment of amyotrophic lateral sclerosis associated with a mutation in the SOD1 gene” (Press release). Food and Drug Administration (FDA). Jump up to: a b New Drug Therapy Approvals 2023 (PDF). Food and Drug Administration (FDA) (Report). January 2024.

































Let's personalize your content