The future of CNS drug development: signs of real progress
Drug Target Review
JULY 21, 2025
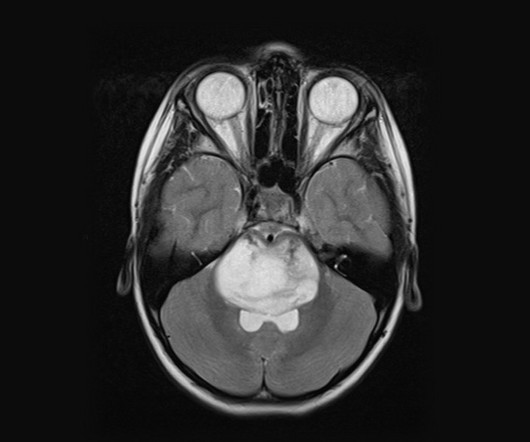
Due to off-target effects, tissue accumulation and immune responses, ASOs face significant challenges, especially for preclinical safety assessments. However, they face challenges in surviving the hostile CNS environment, integrating into existing neural circuits and avoiding immune responses; but they are rich in potential.







































Let's personalize your content