Overcoming challenges in biosimilar analytical characterization
Drug Patent Watch
DECEMBER 9, 2024
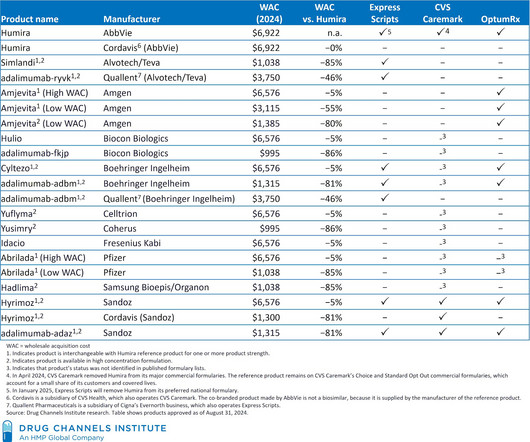
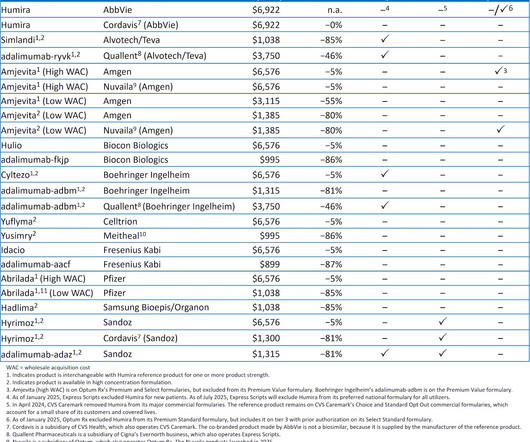
Biosimilars have faced significant challenges in their analytical characterization. This process involves a comprehensive analysis of the biosimilar’s molecular structure, biological activity, and other quality characteristics to demonstrate similarity to the reference product.















































Let's personalize your content