Time for change: non-human primates in drug research
Drug Target Review
JULY 7, 2025
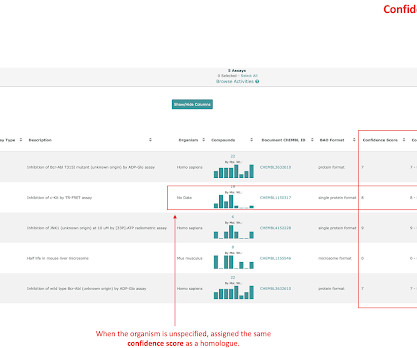
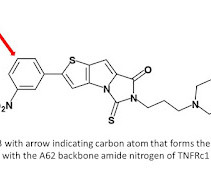
7 This initiative forms part of a broader effort to modernise drug development and align regulatory expectations with emerging new approach methodologies (NAMs), including human cell-based assays, artificial intelligence models and organ-on-chip platforms. In silico toxicology: computational methods for predicting toxicity.


















Let's personalize your content