De-risking drug discovery with predictive AI
Broad Institute
JULY 17, 2024
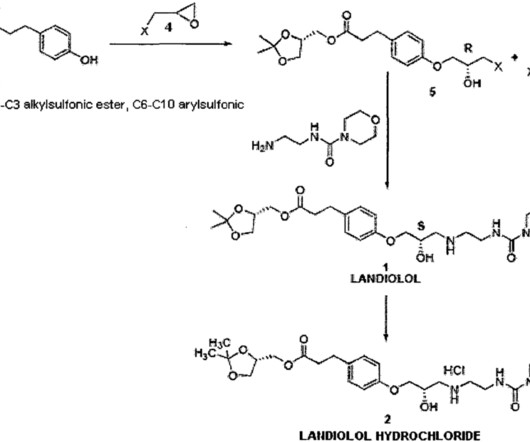
Still, more than 90 percent of drug candidates fail in clinical trials, with even more that never make it to the clinical stage. Together, the tools estimate how a drug may impact diverse outcomes of interest to drug developers: general cellular health, pharmacokinetics, and heart and liver function.
































Let's personalize your content