Allosteric Covalent Inhibitors of the STAT3 Transcription Factor from Virtual Screening
Covalent Modifiers
JUNE 12, 2025
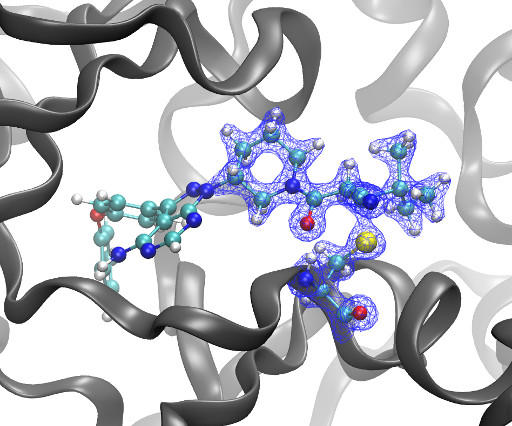
4c00622 The STAT family of transcription factors are important signaling hubs, with several of them, particularly STAT3, being emerging oncotargets already investigated in clinical trials. Keserű ACS Medicinal Chemistry Letters 2025 16 (6), 991-997 DOI: 10.1021/acsmedchemlett.4c00622










































Let's personalize your content