Over The Air Updates for React Native Apps
Perficient: Drug Development
JUNE 2, 2025
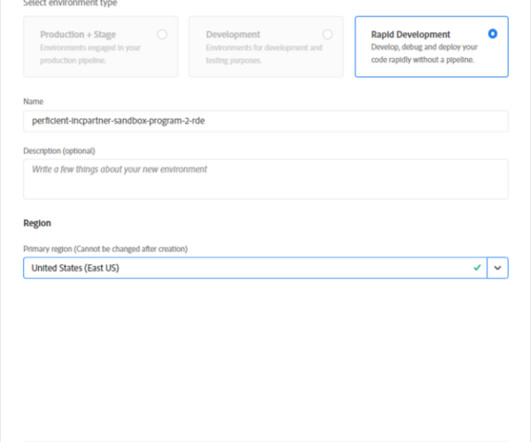
Expo provides a great documentation on setup and configuration. package which added the following dependencies. Test: Unlike App center, Expo provides the same package for iOS and Android targets. The targeted version package is available on the expo server. It is caused by the react-native-elements package.












































Let's personalize your content