A Covalent Self-Reporting Peptide Degrader Enables Real-Time Monitoring of Targeted Protein Degradation In Vivo
Covalent Modifiers
AUGUST 1, 2025
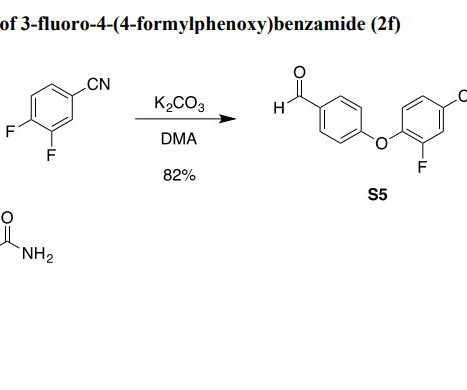
5c07041 Peptides have demonstrated great potential in drug development. However, their broader application in modalities such as proteolysis-targeting chimeras (PROTACs) remains limited by the lack of real-time efficacy feedback and poor pharmacokinetic stability.










































Let's personalize your content