A Visual Guide to Gene Delivery
Codon
JULY 28, 2025
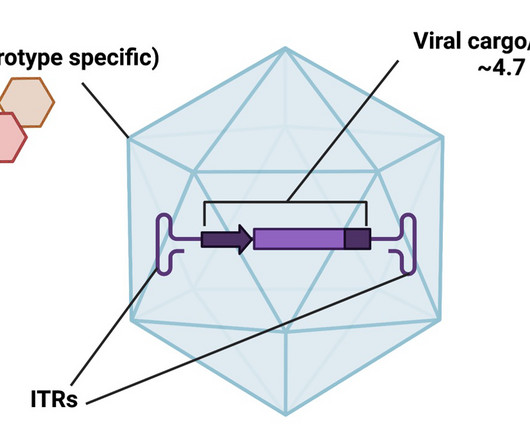
For the former, clinicians extract a patient’s cells, engineer them in a laboratory, and then infuse them back into the body. Some diseases are caused by mutations in large genes that exceed the packaging limits of existing vectors like adeno-associated virus (AAV). Vectors also differ in the duration of their expression.









































Let's personalize your content