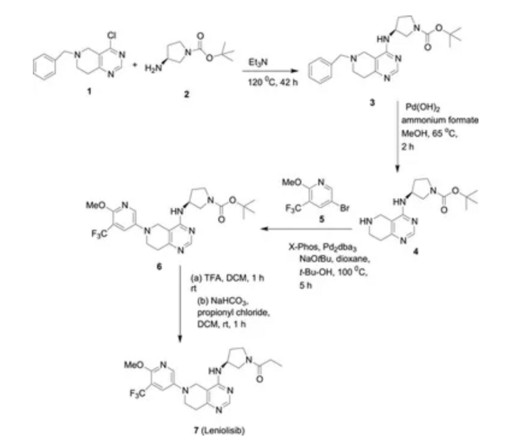
Leniolisib
New Drug Approvals
MAY 15, 2025
2017, 8, 9, 975980 [link] The predominant expression of phosphoinositide 3-kinase (PI3K) in leukocytes and its critical role in B and T cell functions led to the hypothesis that selective inhibitors of this isoform would have potential as therapeutics for the treatment of allergic and inflammatory disease. World Health Organization (2017).















































Let's personalize your content