Time for change: non-human primates in drug research
Drug Target Review
JULY 7, 2025
Persistent challenges with NHP use Despite their biological relevance, using live NHPs in research poses several major challenges: Ethical concerns NHPs, due to their advanced cognition and social behaviour, are at the centre of ongoing ethical debates. Supply chain disruptions Availability is a critical bottleneck in NHP-based research.



















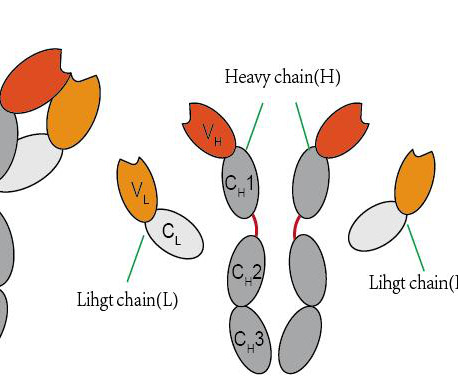











Let's personalize your content