Bayer Expands Collaboration with Tsinghua University to Boost Pharma R&D in China
The Pharma Data
JUNE 24, 2025
Professor Hongwei Wang , Vice-President of Tsinghua University and Bayer Endowed Chair 2018, added: “Over the past sixteen years, Tsinghua University and Bayer have forged a trusted and synergistic partnership that has continuously advanced the integration of scientific discovery and pharmaceutical innovation.






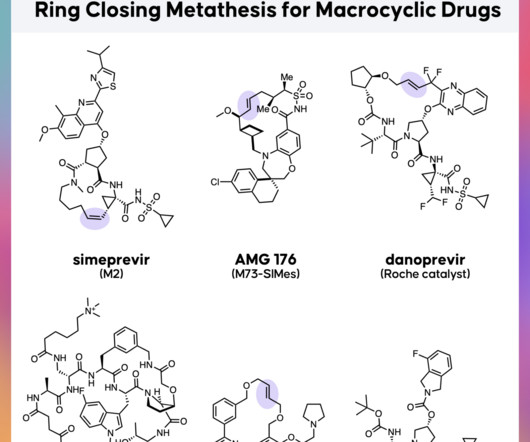






































Let's personalize your content