Vamorolone
New Drug Approvals
JULY 25, 2025
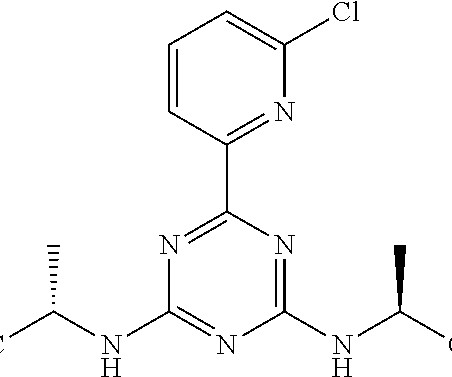
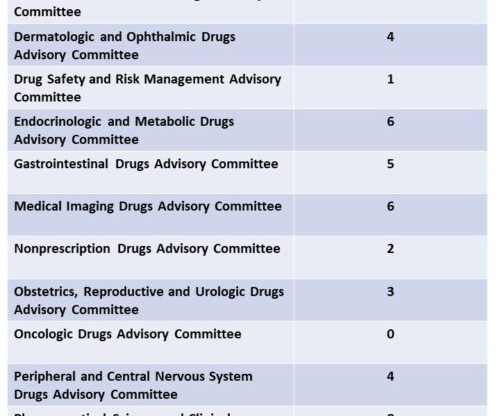
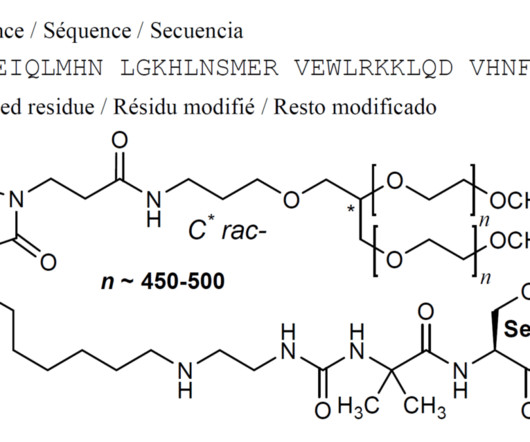
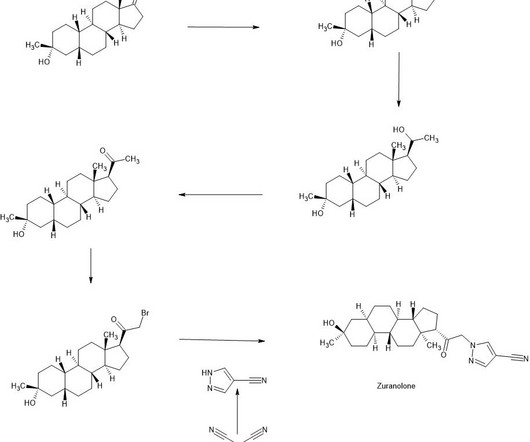
Syn EuropeanJournalofMedicinalChemistry265(2024)116124 Vamorolone (Agamree) On October 26, 2023, Vamorolone, developed jointly by Santhera Pharmaceuticals and ReveraGen BioPharma, has received FDA approval to treat DMD in patients aged 2 years and older [1]. 2018, 38, 320−324. (74) 2018, 38, 320−324. June 2018).







































Let's personalize your content