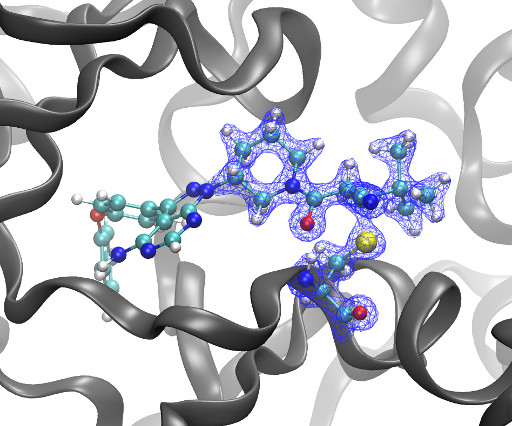
Site-Specific Molecular Glues for the 14-3-3/Tau pS214 ProteinProtein Interaction via Reversible Covalent Imine Tethering
Covalent Modifiers
FEBRUARY 10, 2025
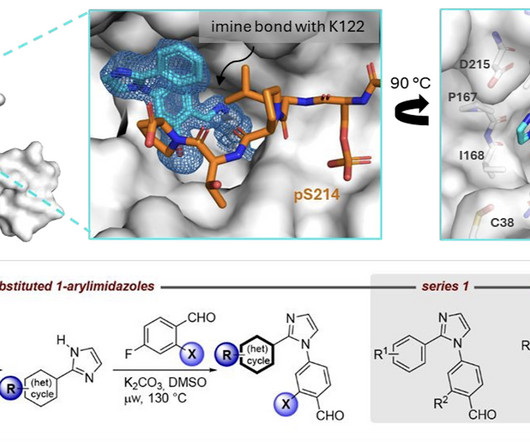
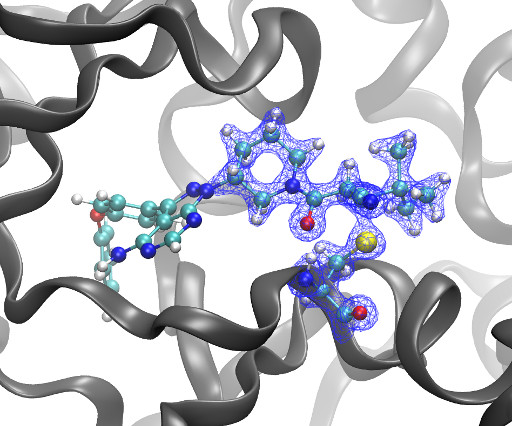
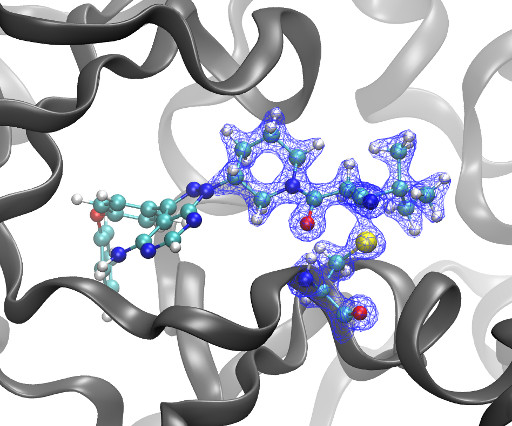
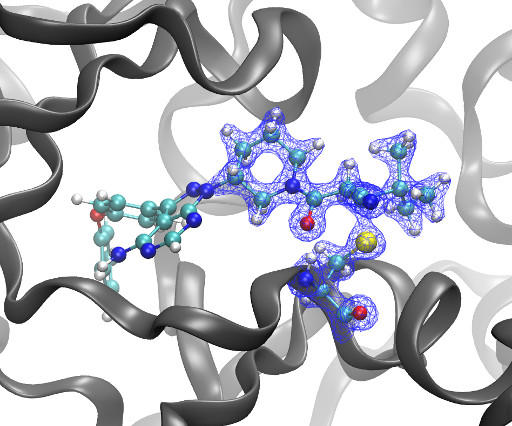
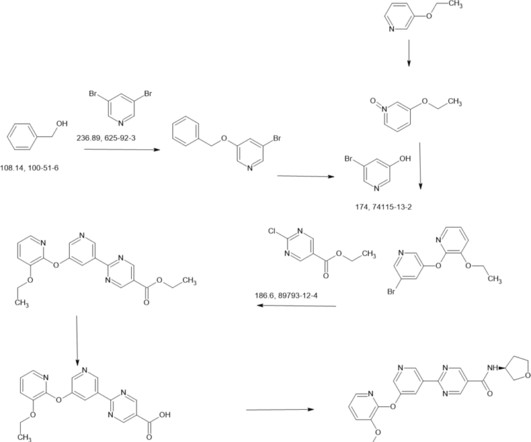
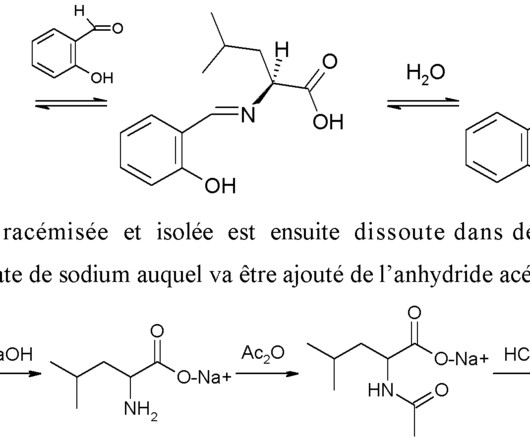
DOI Ansgar Oberheide, Maxime van den Oetelaar, Jakob Scheele, Jan Borggrfe, Semmy Engelen, Michael Sattler, Christian Ottmann, Peter Cossar and Luc Brunsveld RSC Med Chem 2025 [link] Protein-protein interactions (PPIs) are key regulators of various cellular processes.








































Let's personalize your content