Pfizer wins FDA approval for its $7B colitis drug
BioPharma Drive: Drug Pricing
OCTOBER 13, 2023
Acquired with the buyout of Arena Pharmaceuticals, Velsipity enters a competitive market for ulcerative colitis pills.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

BioPharma Drive: Drug Pricing
OCTOBER 13, 2023
Acquired with the buyout of Arena Pharmaceuticals, Velsipity enters a competitive market for ulcerative colitis pills.

BioPharma Drive: Drug Pricing
AUGUST 29, 2023
A broadened clearance for Reblozyl in myelodysplastic syndromes should help Bristol Myers offset the looming loss of revenue from top-selling medicines set to soon lose market exclusivity.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.
Drugs.com
NOVEMBER 17, 2023
16, 2023 -- The first home test for chlamydia and gonorrhea will soon hit the market, following its approval Wednesday by the U.S. Food and Drug Administration. THURSDAY, Nov. People will be able to buy the Simple 2 Test over-the-counter at a.

BioPharma Drive: Drug Pricing
AUGUST 25, 2023
According to one analyst, the approval was the last hurdle keeping Sandoz’s Tyruko from directly competing in the U.S. market against Biogen’s inflammation-regulating medicine Tysabri.

Drugs.com
JANUARY 17, 2025
Food and Drug Administration has authorized the marketing of 20 ZYN nicotine pouch products. FRIDAY, Jan. 17, 2025 -- Following an extensive scientific review, the U.S. Nicotine pouches -- small synthetic fiber pouches containing nicotine -- are.
BioPharma Drive: Drug Pricing
AUGUST 5, 2023
The agency turned back the companies' attempt to also win clearance for major depressive disorder, limiting its market potential.

Drugs.com
JUNE 13, 2023
Food and Drug Administration has approved over-the-counter marketing for the product, called Eroxon, as a. TUESDAY, June 13, 2023 -- Men with erectile dysfunction will now have the option of using a topical gel to treat the condition.

Drugs.com
APRIL 1, 2025
Food and Drug Administration granted marketing authorization for the first home-based, nonprescription diagnostic test for chlamydia, gonorrhea, and trichomoniasis in women, the agency announced Friday.Women with. TUESDAY, April 1, 2025 -- The U.S.

BioPharma Drive: Drug Pricing
JUNE 16, 2023
The approval of Columvi adds another “bispecific antibody” to the market, highlighting the fast research progress for drugs that target two proteins rather than one.

Drugs.com
JUNE 13, 2023
Food and Drug Administration has approved over-the-counter marketing for the product, called Eroxon, as a. TUESDAY, June 13, 2023 -- Men with erectile dysfunction will now have the option of using a topical gel to treat the condition.

The Pharma Data
JUNE 11, 2025
FDA Approves Tablet Formulation of BeOne’s BRUKINSA® for All Approved Indications, Offering Greater Convenience for Patients with B-cell Cancers BeOne Medicines Ltd. Food and Drug Administration (FDA). a global oncology-focused biopharmaceutical company, has received a significant regulatory milestone from the U.S.

The Pharma Data
JUNE 9, 2025
FDA Approves Merck’s ENFLONSIA™ to Protect Infants from Severe RSV Illness Merck operating as MSD outside the United States and Canada, has received a significant regulatory milestone with the U.S. Food and Drug Administration (FDA) granting approval for ENFLONSIA™ (clesrovimab-cfor).

Drugs.com
JUNE 13, 2023
Food and Drug Administration has approved over-the-counter marketing for the product, called Eroxon, as a. TUESDAY, June 13, 2023 -- Men with erectile dysfunction will now have the option of using a topical gel to treat the condition.

Drug Patent Watch
APRIL 29, 2025
"Market Access: The Hidden Hurdle in Generic Drug Commercialization As a seasoned pharmaceutical professional, I've seen it time and time again: a generic drug finally receives FDA approval, only to stall in the market due to a seemingly insurmountable obstacle - market access.

Drug Patent Watch
APRIL 8, 2025
Patient Safety in the Era of Generic Drugs: A Call to Action As the pharmaceutical industry continues to evolve, one thing remains constant: the need for patient safety. With the rise of generic drugs, it's more important than ever to ensure that these alternatives meet the same high standards as their brand-name counterparts.

SCIENMAG: Medicine & Health
JULY 26, 2023
July 26, 2023 – Octapharma USA today announced that Balfaxar® (prothrombin complex concentrate, human-lans; marketed in Europe and Canada as octaplex®) has received U.S. PARAMUS, N.J.,
Broad Institute
JULY 17, 2024
Still, more than 90 percent of drug candidates fail in clinical trials, with even more that never make it to the clinical stage. Many drugs fail because they simply aren’t safe. Seal began this work after wondering if more toxicology insights could be gleaned from a drug candidate’s chemical structure.
Drug Patent Watch
AUGUST 12, 2020
Just because a drug has received FDA approval does not mean that it is available in the marketplace. The post Generic Drugs Approved but not Launched – How to Tell When Generic Drugs Will hit the Market appeared first on DrugPatentWatch - Make Better Decisions.
New Drug Approvals
JUNE 11, 2025
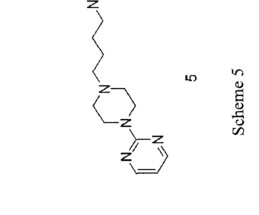
2] Gepirone was synthesized by Bristol-Myers Squibb in 1986 and was developed and marketed by Fabre-Kramer Pharmaceuticals. [4] 4] It was approved for the treatment of major depressive disorder in the United States in September 2023. [4] 12] However, in March 2016, the FDA reversed its decision and gave gepirone ER a positive review.

The Pharma Data
JANUARY 21, 2021
Food and Drug Administration (FDA) gave a greenlight for ViiV Healthcare ’s Cabenuva. Cabotegravir is a ViiV product marketed as Cabenuva, and rilpivirine is a Janssen product with a brand name Edurant. Cabotegravir is a ViiV product marketed as Cabenuva, and rilpivirine is a Janssen product with a brand name Edurant.

ASPET
OCTOBER 12, 2023
To maintain cadence with looming threats in a prolonged field care environment, the broader medical countermeasure (MCM) enterprise must adopt new strategies for CBRN-addressing drug development. CBRN MCMs).

SCIENMAG: Medicine & Health
NOVEMBER 16, 2023
Over the last four decades, insulin manufacturers have extended their periods of market exclusivity on brand-name insulin products by employing several strategies, including filing additional patents on their products after FDA approval and obtaining many patents on delivery devices for their insulin products.

The Pharma Data
NOVEMBER 27, 2020
FDA Approves Danyelza (naxitamab-gqgk) for the Treatment of Neuroblastoma. Food and Drug Administration (“FDA”) has approved Danyelza (naxitamab-gqgk) 40mg/10ml. The product has received Priority Review, Orphan Drug, Breakthrough Therapy, and Rare Pediatric Disease designations from the FDA.

The Pharma Data
DECEMBER 15, 2020
FDA Approves Klisyri (tirbanibulin) for the Treatment of Actinic Keratosis on the Face or Scalp. Food and Drug Administration (FDA) has approved Klisyri (tirbanibulin) for the topical treatment of actinic keratosis (AK) on the face or scalp. The FDA approval of Klisyri is a significant milestone for Athenex.

The Pharma Data
NOVEMBER 2, 2020
FDA Approves Bronchitol (mannitol) Inhalation Powder to Improve Pulmonary Function in Adult Patients with Cystic Fibrosis. Food and Drug Administration (FDA) approval of Bronchitol (mannitol) inhalation powder. Food and Drug Administration (FDA) approval of Bronchitol (mannitol) inhalation powder.
The Pharma Data
AUGUST 31, 2021
Food and Drug Administration (FDA) has converted this indication from an accelerated to a full (regular) approval. 10), as determined by an FDA-approved test, or in patients who were not eligible for any platinum-containing chemotherapy regardless of PD-L1 status.
New Drug Approvals
MAY 8, 2025
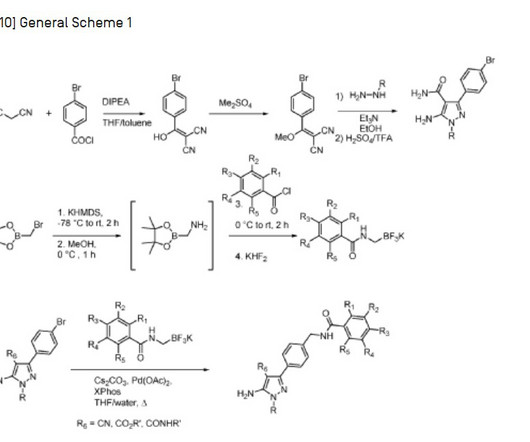
7] Pirtobrutinib was approved for medical use in the United States in January 2023, [4] [8] [9] [10] and in the European Union in November 2023. [2] 14] Pirtobrutinib was approved for medical use in the European Union in November 2023. [2] 14] Pirtobrutinib was approved for medical use in the European Union in November 2023. [2]
The Pharma Data
NOVEMBER 2, 2020
Food and Drug Administration, Gover said he believes there is a “clear path” to the submission of a New Drug Application as soon as 2021. If nabiximols is approved, this would mark the second cannabis-based product for GW Pharmaceuticals in the United States. Now is the ideal time to develop nabiximols in the U.S.

The Pharma Data
NOVEMBER 27, 2020
FDA Approves Imcivree (setmelanotide) for Chronic Weight Management in Patients with Obesity Due to POMC, PCSK1 or LEPR Deficiency. With this approval, Imcivree becomes the first-ever FDA approved therapy for these rare genetic diseases of obesity. BOSTON, Nov. in the first quarter of 2021.

The Pharma Data
OCTOBER 30, 2020
To increase demand for products during the novel coronavirus pandemic, the US Food and Drug Administration (FDA) has already approved at-home testing and investigated the effectiveness of malaria pills and other antiviral drugs to treat Covid-19 9. FDA approves first treatment for Covid-19.
Drug Target Review
JUNE 5, 2025
Dr Aaron Haubner, Senior Manager of North America Medical Affairs and Market Access at Terumo Blood and Cell Technologies , reveals that while promising new treatments emerge, urgent partnerships are needed to ensure this essential blood therapy reaches the patients who need it most. JAMA Netw Open. 2023 Nov 1;6(11):e2344546. 2023.44546.

The Pharma Data
MAY 19, 2023
ABBOTT RECEIVES FDA APPROVAL FOR TACTIFLEX™ ABLATION CATHETER FOR TREATMENT OF ABNORMAL HEART RHYTHM Abbott (NYSE: ABT) today announced that the U.S. Unlike other catheters on the market, the TactiFlex catheter uses a tip design with a laser-cut pattern that flexes when in contact with the heart wall.

Advarra
JULY 26, 2022
However, it’s not legal federally and is considered a Schedule I drug by the U.S. Drug Enforcement Administration (DEA), meaning it has no accepted medical use and a high potential for abuse. With the exception of one highly purified form of CBD (Epidiolex), no other CBD products are currently FDA approved.

FDA Law Blog: Biosimilars
OCTOBER 13, 2024
Designed to incentivize the development of drugs for pediatric rare diseases where such development may not otherwise have occurred, vouchers may be granted for drugs for serious or life-threatening rare diseases where the serious or life-threatening manifestations primarily affect individuals aged from birth to 18 years.

The Pharma Data
OCTOBER 31, 2020
Food and Drug Administration (“FDA”) has approved the Investigation New Drug (IND) application for TG-1000, a novel treatment for influenza A and B. ” According to Global Data, the global market for influenza antivirals reached 2.34 TAIPEI, Taiwan , Nov. billion USD by 2026 at a CAGR of 11.5%.
New Drug Approvals
APRIL 18, 2025
2] Crinecerfont was approved for medical use in the United States in December 2024. [2] 2] [3] The US Food and Drug Administration (FDA) considers it to be a first-in-class medication. [4] 2] Crinecerfont was approved for medical use in the United States in December 2024. [2] Food and Drug Administration (FDA).

The Pharma Data
JUNE 28, 2023
FDA Approves Pfizer’s NGENLA™, a Long-Acting Once-Weekly Treatment for Pediatric Growth Hormone Deficiency NEW YORK & MIAMI–(BUSINESS WIRE)– Pfizer Inc. NGENLA is approved for the treatment of pediatric GHD in more than 40 markets including Canada, Australia, Japan, and EU Member States.

The Pharma Data
OCTOBER 21, 2020
a biopharmaceutical company developing multiple assets in the ophthalmic and injectable areas, announced today it received approval from the U.S. Food and Drug Administration (FDA) to market Ephedrine Sulfate Injection in a ready-to-use 50mg/10 ml single use vial presentation. BRIDGEWATER, N.J., and Canada.
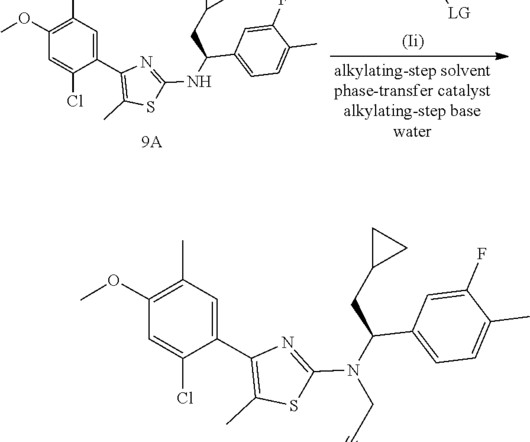
The Pharma Data
AUGUST 19, 2021
today announced that the company’s Marketing Authorization Application (MAA) for lenacapavir, an investigational, long-acting HIV-1 capsid inhibitor, has been fully validated and is now under evaluation with the European Medicines Agency (EMA). In June 2021, Gilead submitted a New Drug Application (NDA) for lenacapavir seeking U.S.
The Pharma Data
AUGUST 20, 2020
The FDA has approved its first generic of Biogen’s multiple sclerosis (MS) treatment Tecfidera, awarding authorisation to Mylan which is launching the drug in a dimethyl fumarate delayed-release oral solid formulation, both in 120mg and 240mg doses. The drug generated $3.79 billion for Biogen through 2019. Matt Fellows.
The Pharma Data
JULY 29, 2021
Food and Drug Administration approved the first interchangeable biosimilar insulin product, indicated to improve glycemic control in adults and pediatric patients with Type 1 diabetes mellitus and in adults with Type 2 diabetes mellitus. Biosimilars marketed in the U.S.

The Pharma Data
MAY 17, 2021
The authority to change drug labels outside of considerations for new safety information “could encourage third parties, such as academic investigators, insurance companies, and cooperative trial groups, to initiate such changes,” they wrote. . Posted 17 May 2021 | By Jeff Craven .

Policy Prescription
OCTOBER 3, 2023
Salant’s paper supports personal drug importation and legalizing the importation of prescription drugs for commercial use so that wholesale pharmacies, including companies like Amazon and Costco, could tap into the parallel importation markets of other countries. First, drug prices in the U.S. He points to “a mere 1.5%

FDA Law Blog: Biosimilars
JUNE 27, 2023
Valentine Named Top Lawyer Under 40; Only Food and Drug Lawyer Selected Hyman, Phelps & McNamara, P.C. (HP&M) James was only one of five life sciences attorneys selected and the only food and drug lawyer to make the list. HP&M’s James E. Valentine , as a 2023 Rising Star.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content