Scientists discover a secret to regulating our body clock, offering new approach to end jet lag
Science Daily: Pharmacology News
OCTOBER 7, 2024
Scientists have discovered the secret to regulating our internal clock.
This site uses cookies to improve your experience. To help us insure we adhere to various privacy regulations, please select your country/region of residence. If you do not select a country, we will assume you are from the United States. Select your Cookie Settings or view our Privacy Policy and Terms of Use.
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.
Used for the proper function of the website
Used for monitoring website traffic and interactions
Cookies and similar technologies are used on this website for proper function of the website, for tracking performance analytics and for marketing purposes. We and some of our third-party providers may use cookie data for various purposes. Please review the cookie settings below and choose your preference.

Science Daily: Pharmacology News
OCTOBER 7, 2024
Scientists have discovered the secret to regulating our internal clock.

Science Daily: Pharmacology News
DECEMBER 6, 2023
Although this had already been observed in the local universe, an international research team has just revealed the existence of the phenomenon in galaxies which are more than 7 billion years old and actively forming stars, the category to which most galaxies belong.
This site is protected by reCAPTCHA and the Google Privacy Policy and Terms of Service apply.

Perficient: Drug Development
NOVEMBER 6, 2024
Artificial intelligence (AI) is poised to affect every aspect of the world economy and play a significant role in the global financial system, leading financial regulators around the world to take various steps to address the impact of AI on their areas of responsibility.

The Premier Consulting Blog
OCTOBER 30, 2024
One of the key facets of the OCET program is providing internal training opportunities to FDA staff, to build their expertise with the novel technologies. Contact us today to find out how we can support your program.

thought leadership
DECEMBER 9, 2024
In the decades since the FDA first introduced Good Manufacturing Practice (GMP) regulations, the drug and device industry has undergone significant transformation. Alongside these innovations, global supply chains have expanded, requiring manufacturers to coordinate with international partners, each subject to unique regulatory standards.

FDA Law Blog: Biosimilars
JANUARY 9, 2025
As part of the Food and Drug Administration Safety and Innovation Act (known as FDASIA) and later under the FDA Reauthorization Act of 2017 (known as FDARA), a drug or medical device can be deemed adulterated if a regulated company delays, denies, or limits an inspection, or refuses to permit an entry or inspection. FD&C Act 501(j).

Agency IQ
MAY 28, 2024
BY COREY JASEPH, MS, RAC The British medical device regulator just issued its promised framework on international recognition.

Advarra
NOVEMBER 14, 2024
In an era where clinical trials are increasingly global, it’s more imperative than ever to leverage international expertise. Enhanced objectivity: A DMC composed of diverse international experts can mitigate potential biases arising from local or regional perspectives. local standards of care).

PPD
JANUARY 2, 2025
The need for FSP support across different functions may arise because the sponsor has chosen to focus its core workforce on critical internal competencies, leaving other in-house functions undeveloped. Using this approach, CROs can integrate single or multiple FSP services into an existing FSO arrangement or from the outset.

Perficient: Drug Development
NOVEMBER 6, 2023
Our team recently built a POC for Internal ChatGPT that offers a secure and efficient way for companies to leverage the power of chatbots while maintaining control over their internal data. What Does Our Internal ChatGPT POC Do? The Internal ChatGPT proof of concept was developed on Azure’s OpenAI chat interface.
Drug Target Review
SEPTEMBER 8, 2023
This receptor, referred to as retinoic acid receptor alpha (RARα), is recognised for its regulation of gene expression within the nucleus. Enabling T cells to respond to threats To grasp the significance of this discovery, it’s useful to envision the internal layout of a T cell.

SCIENMAG: Medicine & Health
JULY 5, 2023
Living organisms possess an internal biological cycle known as the circadian clock, enabling them to adapt to environmental changes arising from the Earth’s rotation. This internal clock regulates vital physiological processes including sleep, metabolism, hormonal fluctuations, body temperature, and blood pressure.

Drug Patent Watch
DECEMBER 10, 2024
Accelerated Time to Market : By leveraging a connected network of internal experts, integrated CDMOs can help eliminate downtime, optimize processes, and minimize errors, ultimately accelerating time to market.

Agency IQ
MAY 28, 2024
regulator lays out proposal for international device and diagnostics recognition The British medical device regulator just issued its promised framework on international recognition. law as the Medical Devices Regulation 2002 (UK MDR 2002). law as the Medical Devices Regulation 2002 (UK MDR 2002).

Agency IQ
MARCH 7, 2024
BY COREY JASEPH, MS, RAC This week, the British regulator MHRA offered a new peek into its planned medical device regulations. In the wake of Brexit, the E.U. no longer recognized products certified in the U.K., but the U.K. still needed CE-marked products.
Advarra
JANUARY 13, 2023
New privacy regulations seem to form every few months, especially with individual U.S. states adopting their own privacy regulations (e.g., This means even if you code the data, the international privacy regulations apply – even if the Health Insurance Portability and Accountability Act (HIPAA) does not.

FDA Law Blog: Biosimilars
FEBRUARY 26, 2025
Claud The ongoing DOGE-led reductions to the federal workforce and recent sweeping policy changes have spawned many questions for compliance officers and quality managers in FDA-regulated companies. If a compliance or quality program exists only as bargaining chip to use with regulators, thats not a formula to instill good habits.

Drug Patent Watch
FEBRUARY 27, 2025
With the rise of international trade and the increasing complexity of patent laws, it's more challenging than ever to safeguard your intellectual property. One key strategy is to conduct thorough market research and stay up-to-date on the latest patent laws and regulations in each country where you plan to operate.

Conversations in Drug Development Trends
OCTOBER 29, 2024
Through relationship-building and increased engagement, regulators aim to improve their understanding of the ‘lived experience’ of patients with rare diseases and hear their top concerns. For example, Voice of the Patient and Patient-Focused Drug Development meetings are focused on one disease or a group of similar diseases.
Agency IQ
SEPTEMBER 1, 2023
EU’s PIC Regulation adds 35 new entries The European Commission’s latest update to the export and import of hazardous chemicals regulation – which implements the Rotterdam Convention on prior informed consent in the EU – includes 27 pesticides and eight industrial chemicals in the list of substances requiring export notification.

Agency IQ
SEPTEMBER 1, 2023
MHRA introduces an expanded international recognition procedure, while planning to sunset the current EC reliance pathway Today, the U.K.’s Background: MHRA’s reliance on European Commission decisions and the intend to expand recognition to additional regulators The official exit from of the U.K. from the E.U.

Perficient: Drug Development
MARCH 27, 2025
Data privacy regulations such as GDPR , HIPAA , and CCPA impose strict requirements on organizations handling personally identifiable information (PII) and protected health information (PHI). However; in regulated industries, their default implementation may introduce compliance risks that must be addressed. What Are Deletion Vectors?

Alta Sciences
AUGUST 21, 2024
Internships at Altasciences: Q&A With Our Summer Interns nbartlett Thu, 08/22/2024 - 04:44 Internships at Altasciences are more than just a stepping stone—they’re a gateway to real-world-experience and professional growth, giving the next generation opportunities to help shape progress in the drug development industry. The work environment.

Perficient: Drug Development
AUGUST 19, 2024
Banks have silos, these silos have been created due to mergers, regulations, entities, risk types, chinese walls, data protection, land laws or sometimes just technological challenges over time. The team analyzing the data warehouses, the data lakes and aiding the analytics will have to have this one major organizational goal in mind.

FDA Law Blog: Biosimilars
NOVEMBER 9, 2023
Food and Drug Administration (FDA) issued a proposed rule that would amend its prior notice regulations to add new information requirements and deadlines. food supply is safe by shifting the focus of federal regulators from responding to contamination to preventing it.

Agency IQ
MARCH 7, 2024
British regulators tease new device regulations in informative live session This week, the British regulator MHRA offered a new peek into its planned medical device regulations. BY COREY JASEPH, MS, RAC | MAR 5, 2024 11:10 PM CST Quick background on medical device regulation in the U.K. post-market regulation here.]
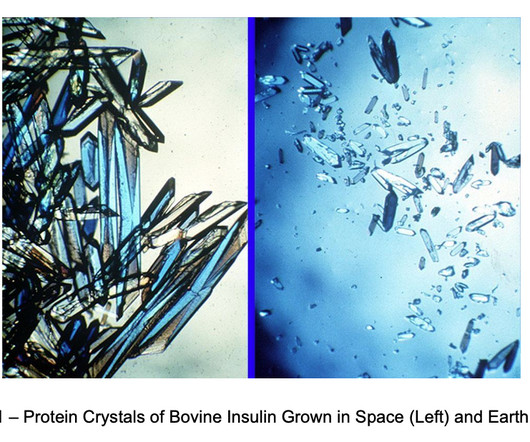
LifeSciVC
MAY 14, 2025
Research continued through the 90s on the Space Shuttle, but only after the 1998 launch of the International Space Station (ISS) did microgravity research achieve sufficient scale and consistency for substantial progress to made in unraveling gravitys effects on molecular and cellular biology. Space.com, 17 Aug. Reichert, Paul et al.

Drug Patent Watch
DECEMBER 30, 2024
Evolving Landscape of New Drug Approval in Japan and Lags from International Birth Dates: Retrospective Regulatory Analysis. Pharmaceutical Regulations in Japan 2020. Clinical Pharmacology & Therapeutics , 111(3), 531538. Freyr Solutions. 11 Must-know FAQs about the Drug Approval Process in Japan.

Drug Target Review
APRIL 7, 2025
For example, transcriptomic processes are showing the potential to identify and track failures in gene expression and gene regulation of amyloid and tau-related biomarkers, understood as precursors to the onset of Alzheimers disease (AD). International Journal of Molecular Sciences. Bagyinszky E, et al. 21(10):3517.

PPD
MAY 7, 2024
The European Union (EU) is on the verge of a significant shift as it prepares to implement new health technology assessment (HTA) regulations in 2025. Challenges and opportunities of the new EU HTA regulation The implementation of centralized HTA presents both opportunities and challenges for pharmaceutical companies.

Agency IQ
AUGUST 18, 2023
and internationally Establishing and communicating a substance’s potential to cause cancer is a cornerstone of chemical regulation worldwide. Explainer: carcinogen classification in the E.U., Given how influential the U.S.,

PPD
DECEMBER 16, 2024
Rising costs have become a persistent challenge for drug developers, driven by a combination of internal and external pressures that have intensified in recent years. Lacking internal expertise or capacity often slows progress, making outsourcing a strategic necessity.

FDA Law Blog: Biosimilars
MAY 4, 2025
Attorneys Office for the Western District of Texas announced that IMC Pro International Inc. 1-BOC-Piperidone is a DEA-regulated List 1 chemical while (2-Bromethyl) benzene is on DEAs longstanding, recently updated Special Surveillance List. (By my count there have been only ten since 2000). On March 26th the U.S.
Perficient: Drug Development
NOVEMBER 27, 2023
In the dynamic environment of highly regulated industries like healthcare and financial services, leaders often balance competing goals to delight customers while cutting costs. This blog was co-authored by Carl Aridas and Joel Thimsen. Build a reliable risk management strategy using accurate estimations and predictions.

Chemical Biology and Drug Design
JANUARY 15, 2024
The expression levels of endoplasmic reticulum (ER) stress-related protein and silent information regulator 1/nuclear factor kappa-B (Sirt1/NF-κB) signaling pathway were used to observe the therapeutic effect of naringin.

FDA Law Blog: Drug Discovery
FEBRUARY 27, 2025
Livornese As anticipated, the International Council for Harmonization (ICH) published the Good Clinical Practice (GCP) guideline E6(R3) Principles and Annex 1 on January 6, 2025. How regulators will interpret and enforce its provisions may not become clear for some time. By Julie Kim & Deborah L.

Advarra
MARCH 6, 2025
Food and Drug Administrations (FDA) regulations on good clinical practice, which are based on ethical standards outlined in frameworks such as the Belmont Report and the Declaration of Helsinki. Advarra ensures that clinical trials comply with the U.S.

PPD
JULY 16, 2024
Staying current with PV-relevant regulations and regulatory guidance can be challenging and often requires an FSP PV RI team that spans multiple countries and languages. This includes everything from clarifications sought from individual regulatory authorities to Q&A documents, other professional publications and conference presentations.

Agency IQ
JUNE 17, 2024
Swiss regulators align with EU on chemicals, biocides The Swiss Notification Authority for Chemicals has announced new adaptations are in the offing that will harmonize parts of the Swiss Chemicals and Biocidal Products ordinances with recent updates to corresponding EU legislation.
DrugBank
APRIL 25, 2024
Overcoming internal resistance to change is also part of the journey, requiring clear demonstration of AI's benefits and involving your team in the transition. Security: Strong cybersecurity is non-negotiable to protect sensitive data and comply with international regulations. In the U.S.,

Agency IQ
AUGUST 9, 2024
Commission proposes exemptions to UV-328 ban under POPs Regulation The European Commission plans to implement exemptions introduced under the Stockholm Convention’s 2023 ban of UV-328, an ultraviolet-light absorbing persistent organic pollutant (POP).

Elrig
MARCH 25, 2025
After PhD and postdoctoral work in the UK and Singapore, Prof Johnson received a prestigious Ramn y Cajal fellowship at the Centre for Genomic Regulation (Barcelona). GOLD Lab collaborates with leading international consortia, including Genomics England and FANTOM (Functional Annotation of the Mammalian Genome).

Agency IQ
JANUARY 12, 2024
device regulation timelines To kick off 2024, the British device regulator offered its medical device and IVD plans for this year and next, promising public action on the post-market surveillance regulation by mid-2024 and on the core regulations in late 2024 or early 2025. New roadmap sets out U.K.

The Premier Consulting Blog
JANUARY 14, 2025
International Projects Project Orbis An initiative for collaboration among international regulators Provide a framework for concurrent submission and review of oncology products among international partners, including Australia, Brazil, Canada, Israel, Singapore, Switzerland, and the UK.
Expert insights. Personalized for you.
We have resent the email to
Are you sure you want to cancel your subscriptions?


Let's personalize your content