Reviewing the IDH1 Mutation‐Mediated Mechanism of Drug Resistance and Revisiting Its Overcoming Strategies
Chemical Biology and Drug Design
APRIL 4, 2025
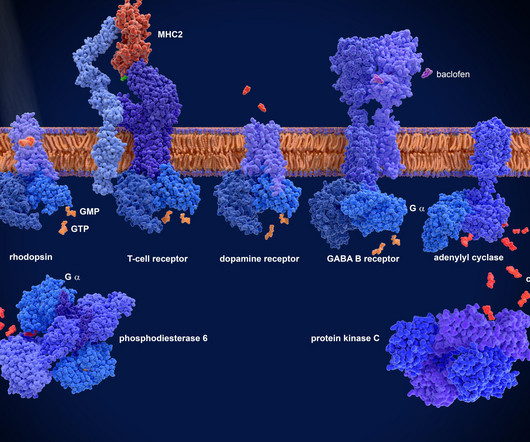
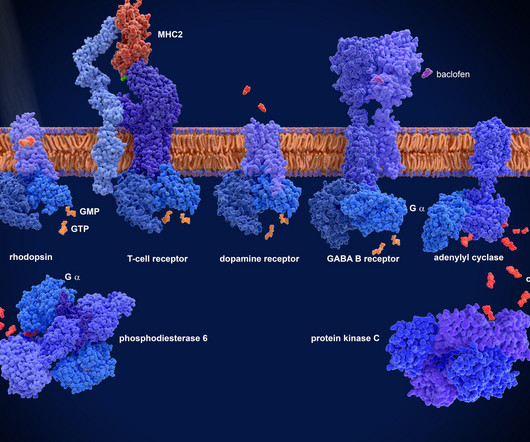
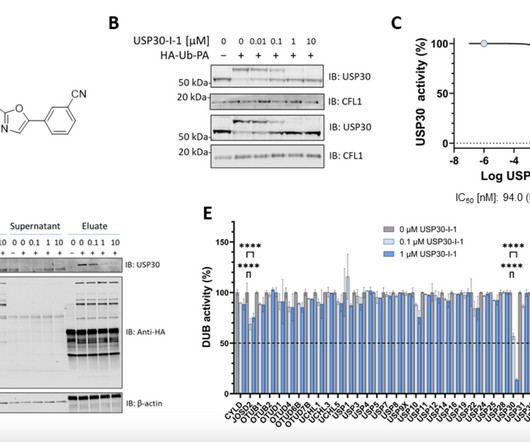
At present, there are few studies on the drug resistance of mIDH1 inhibitors. Currently, the FDA has granted approval for the use of the small molecule inhibitor Ivosidenib (AG-120) in the treatment of IDH1-mutated AML and cholangiocarcinoma. Representative mIDH1 inhibitors and their binding modes were also discussed.





































Let's personalize your content