Vamorolone
New Drug Approvals
JULY 25, 2025
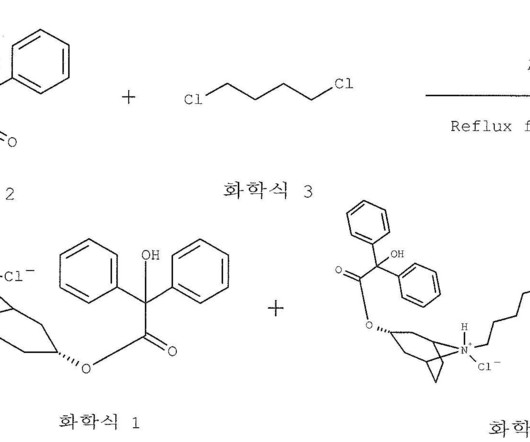
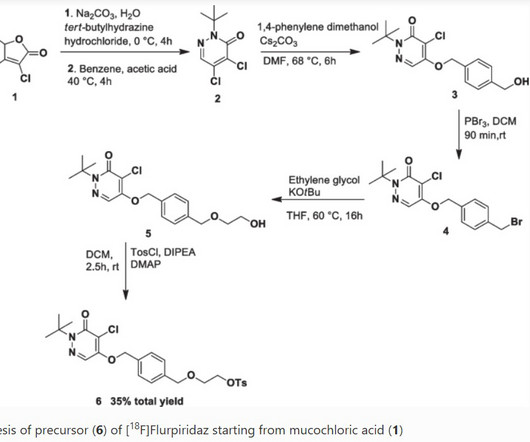
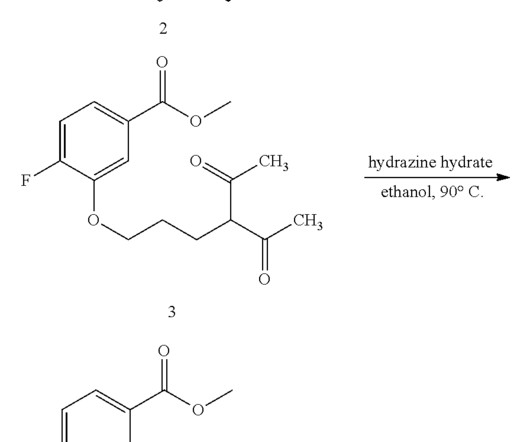
The synthesis is disclosed in Bioorganic & Medicinal Chemistry, Volume 21 , Issue 8, 15 April 2013, Pages 2241-2249. Hoffman, Efficacy and safety of vamorolone vs placebo and prednisone among boys with duchenne muscular dystrophy: a randomized clinical trial, JAMA Neurol. 21 (2013) 2241–2249. 2013, 21, 2241− 2249.





































Let's personalize your content