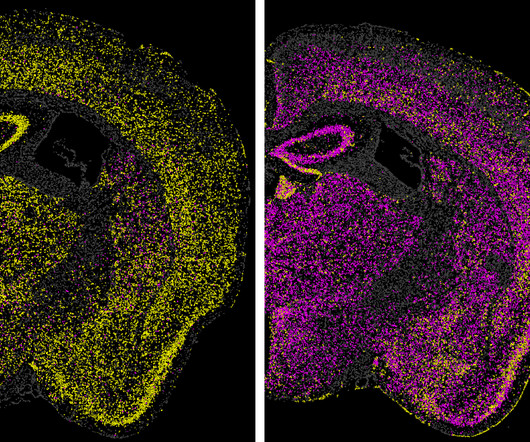
Levers for Biological Progress
Codon
NOVEMBER 1, 2024
” Nobody really knows without trying it out in the laboratory. Though my focus in this essay is narrow — I don’t discuss bottlenecks in clinical trials, human disease, or animal testing — I hope others will take on these challenges in similar essays. Subscribe to Asimov Press. There are a few reasons.















Let's personalize your content