High Throughput Screening (HTS)
Sygnature Discovery
MARCH 14, 2025
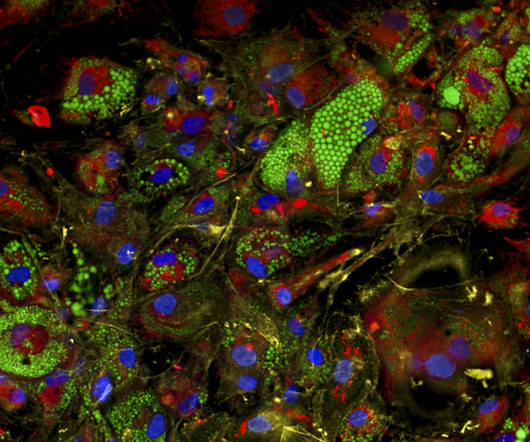
High Throughput Screening Workflow The HTS Workflow is tailored to each of our clients requirements, focusing on developing robust assays with a flexible approach, especially in selecting hits to balance false positives and negatives. The process begins with assay development or transfer.




















Let's personalize your content