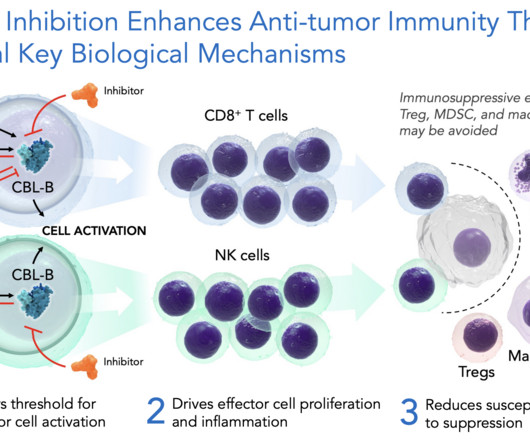
Why radiopharmaceuticals are gaining ground in the fight against cancer
Drug Target Review
JUNE 6, 2025
At the forefront of this transformation is Dr Ebrahim Delpassand, a nuclear medicine physician and the driving force behind RadioMedix (RMX), a radiopharmaceutical company focused on developing targeted diagnostics and therapies. These measures ensure both the efficacy and safety of the final product.





































Let's personalize your content