The rising impact of biomarkers in early clinical development
Drug Target Review
APRIL 7, 2025
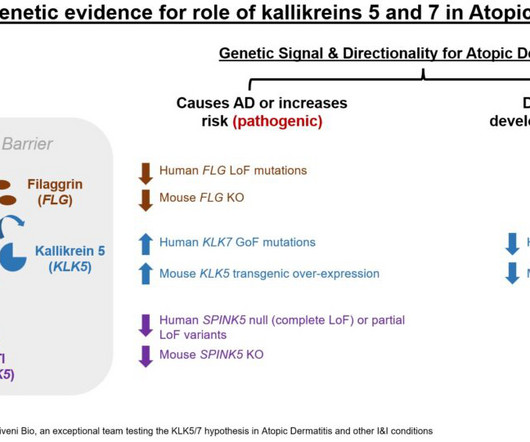
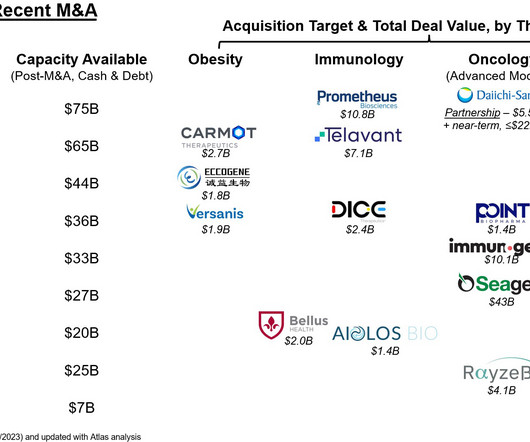
Investigational products with novel mechanisms of action are also assessed for safety in unique ways, creating complexities that can be more dynamically and effectively monitored using biologically relevant biomarkers. Biomarkers can play a crucial role throughout clinical development, especially in early phases.










































Let's personalize your content