Histidine-Covalent Stapled Alpha-Helical Peptides Targeting hMcl-1
Covalent Modifiers
MAY 5, 2024
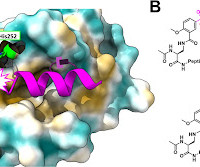
Giulia Alboreggia, Parima Udompholkul, Carlo Baggio, Kendall Muzzarelli, Zahra Assar, and Maurizio Pellecchia Journal of Medicinal Chemistry 2024 DOI: 10.1021/acs.jmedchem.4c00277 Several novel and effective cysteine targeting (Cys) covalent drugs are in clinical use. However, the target area containing a druggable Cys residue is limited. Therefore, methods for creating covalent drugs that target different residues are being looked for; examples of such ligands include those that target the resi










Let's personalize your content