Designing antibodies to think before they bind
Drug Target Review
JULY 24, 2025
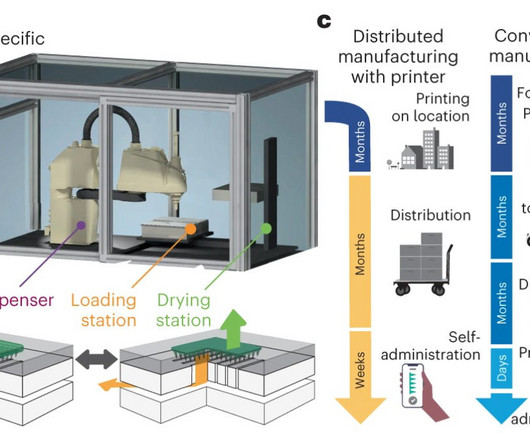
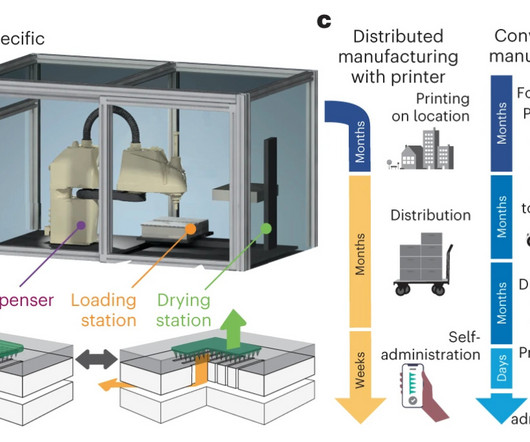
Engineering against resistance: the case of AP402 HER2-positive breast cancer already presents a therapeutic challenge, marked by aggressive tumour growth driven by overexpression of the HER2 receptor. The outcome is a bispecific designed to overcome resistance and activate the immune system precisely where it is needed.




























Let's personalize your content