SpliceBio lands $135M for a new kind of eye gene therapy
BioPharma Drive: Drug Pricing
JUNE 11, 2025
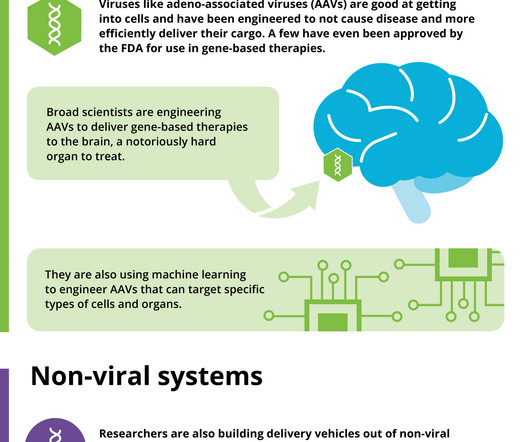
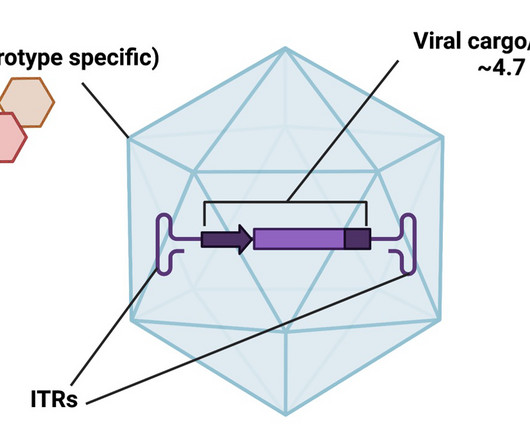
The startup, which is backed by the venture arms of Sanofi, Roche and Novartis, is using dual adeno-associated viruses to help overcome the packaging constraints of current genetic medicines.




































Let's personalize your content