PRITELIVIR MESYLATE
New Drug Approvals
JULY 8, 2025
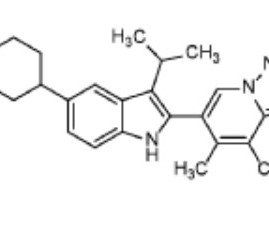
1428321-10-1 Pritelivir mesylate is an antiviral drug currently under development, specifically targeting herpes simplex virus types 1 and 2 (HSV-1 and HSV-2). Clinical Trials: Pritelivir is currently in phase II clinical trials, with ongoing research into its effectiveness and safety.










































Let's personalize your content