Tofersen
New Drug Approvals
MAY 17, 2025
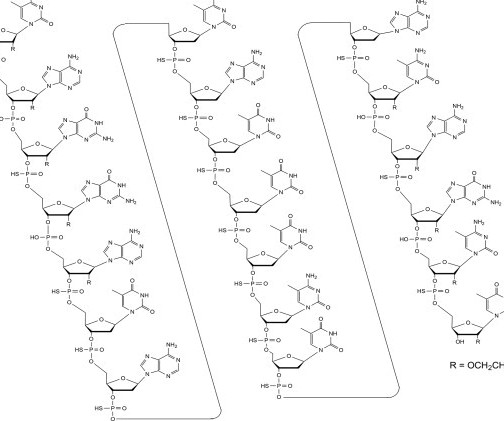
3 November 2006. It is administered as an intrathecal injection. [3] 3] The most common side effects include fatigue, arthralgia (joint pain), increased cerebrospinal (brain and spinal cord) fluid white blood cells, and myalgia (muscle pain). [3] 4] The US Food and Drug Administration (FDA) considers it to be a first-in-class medication. [7]


























Let's personalize your content