The rising impact of biomarkers in early clinical development
Drug Target Review
APRIL 7, 2025
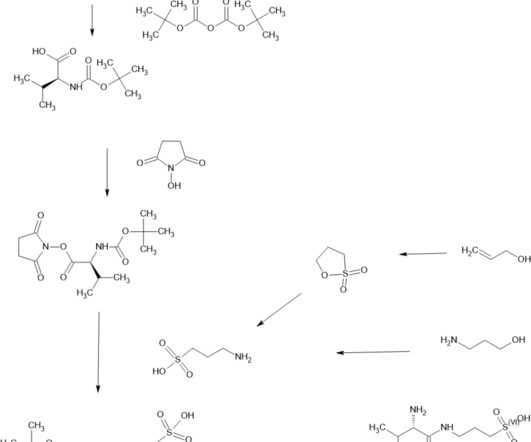
Prior to being validated, the FDA will still consider biomarkers in the marketing approval process as a reasonably likely surrogate endpoint or candidate surrogate endpoint. Silver Spring (MD): Food and Drug Administration (US); 2016-. References FDA-NIH Biomarker Working Group. Bagyinszky E, et al. 21(10):3517.











































Let's personalize your content