Scientists apply optical pooled CRISPR screening to identify potential new Ebola drug targets
Broad Institute
JULY 24, 2025
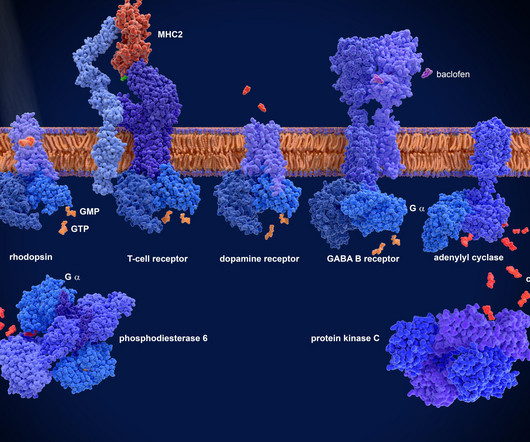
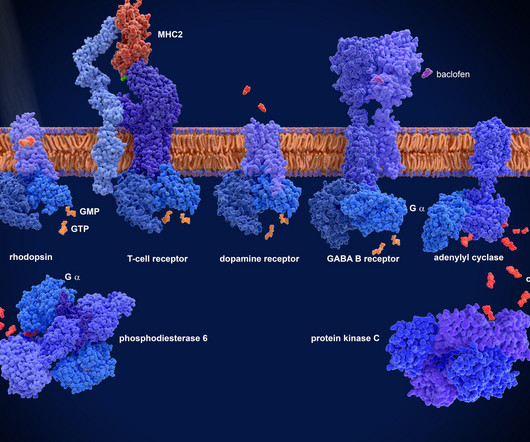
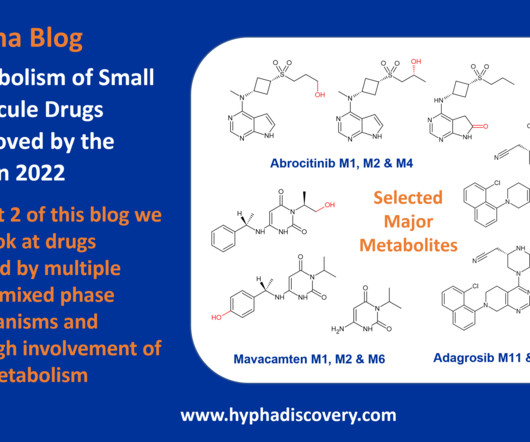
Murphy New treatments are sorely needed for Ebola virus infections, which cause rare, yet severe, outbreaks. Related news Imaging combined with genetic screening of cells enhances genomic discoveries Although outbreaks of Ebola virus are rare, the disease is severe and often fatal, with few treatment options.





































Let's personalize your content